Article Text
Abstract
Background A previous analysis examined the contribution of endocrine disruptor exposures (endocrine-disrupting chemicals, EDCs) to adult diabetes, but was limited to effects of phthalates in middle-aged women and did not simultaneously examine multiple EDCs which are known to coexist in the environment. We therefore endeavoured to quantify potential reductions in diabetes and disease costs that could result from reducing synthetic chemical diabetogenic exposures in the elderly in Europe.
Methods We leveraged the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study (∼1000 participants), which has measured exposure to phthalates; dichlorodiphenyltrichloroethylene; polychlorinated biphenyls (PCBs) and perfluoroalkyl substances to examine their independent contribution to diabetes. We estimated risk reductions assuming identical 25% reductions across levels of 4 selected compounds (PCB 153, monoethylphthalate, dichlorodiphenyldichloroethylene and perfluorononanoic acid), and diabetes costs saved in European men and women if diabetogenic exposures are limited.
Results Reduction of chemical exposures was associated with a 13% (95% CI 2% to 22%) reduction in prevalent diabetes, compared with 40% resulting from an identical (25%) reduction in body mass index (BMI) in cross-sectional analyses. Extrapolating to Europe, 152 481 cases of diabetes in Europe and €4.51 billion/year in associated costs could be prevented, compared with 469 172 cases prevented by reducing BMI.
Conclusions These findings support regulatory and individual efforts to reduce chemical exposures to reduce the burden and costs of diabetes.
- DIABETES
- ENVIRONMENTAL HEALTH
- Epidemiological methods
- Economic evaluation
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Introduction
Increasing evidence suggests that synthetic chemicals commonly found in the environment contribute to metabolic disorders, especially obesity and diabetes.1 ,2 Though diet and physical activity are the major contributors, chemical exposures can be regulated. The costs of safer alternatives to the diabetogenic and other metabolic disruptors can be compared with the health benefits of prevention.3
A recent report suggests that €15 billion in costs of new-onset, type 2 diabetes in older women are attributable to endocrine-disrupting chemicals (EDCs).4 This study leveraged the Nurses’ Health Study which associated urinary phthalates with longitudinal increases in diabetes, controlling many relevant confounders. Though this study did control for another plausible diabetogen, bisphenol A, the study was unable to control for persistent organic pollutants that coexist and may have supra-additive effects.5
We therefore examined data from the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study, which measured persistent and non-persistent chemical exposure to examine their independent contribution to diabetes. Previous publications have associated prevalent diabetes with polychlorinated biphenyls (PCBs), persistent chlorinated pesticides, phthalates and perfluoroalkyl substances (PFASs).6–9 Use of PIVUS also permits quantification of attributable burden in men as well as women, in whom exposures are likely to induce diabetes independent of sex steroid disruption, since the age is 70 years in all participants.
To compare risks with other common risks, we modelled identical 25% percentage reductions in contaminant levels, as well as in body mass index (BMI). We also examined the aggregate reduction in risk produced by simultaneously reducing all four contaminants to assess an aggregate burden of diabetes that can be attributed to environmental contaminants. Finally, we leveraged cost-of-illness data to estimate the preventable cost of adult diabetes in Europeans.
Methods
Sample
PIVUS is a population-based cohort derived from the individuals aged 70 living in the city of Uppsala, Sweden (n=1016; 50% women). For full details, please see Lind et al.10 Prevalent diabetes was defined as antidiabetic therapy or fasting plasma glucose ≥7.0 mmol/L (n=119). Fasting blood was drawn in the morning for the determination of 33 environmental contaminants. The following calculations were based on data previously presented regarding the risk of prevalent diabetes of different environmental contaminants.6–9 The present analyses use a cross-sectional design. Owing to randomly missing data for some of the contaminants, data from 953 of the participants were used in the calculations.
Statistical methods
Biomarkers were selected based on significance in multivariable, single-exposure models: plasma monoethylphthalate (MEP); serum dichlorodiphenyldichloroethylene (p,p′-DDE); serum 2,2′,4,4′,5,5’-hexachlorobiphenyl (PCB 153); and perfluorononanoic acid (PFNA). The analytical procedures have previously been given in detail (6–9). Poisson regression models were used as the bases for the calculations. Included as independent variables in the models were the four contaminants as well as sex, BMI, physical activity, daily energy intake and daily alcohol intake. Population attributable fractions (PAFs) were calculated based on the attribrisk function in the R-package with the same name with two exceptions. Attribrisk uses logistic regression whereas we sought relative risk/prevalence ratio as the outcome could be considered common and an OR would not be a good approximation for the relative risk. Hence, Poisson regression was used instead, following published methods.11 To estimate PAFs, we first note that the estimated probability from the Poisson regression of being a case for any individual in the sample is
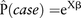 where Xβ is the linear predictor resulting from the regression model. X denotes the design matrix and β is the vector of regression coefficient. Focusing on cases only, we can estimate the probability of being a case under a hypothetical scenario in which the exposure(s) are reduced by a certain amount given as
where Xβ is the linear predictor resulting from the regression model. X denotes the design matrix and β is the vector of regression coefficient. Focusing on cases only, we can estimate the probability of being a case under a hypothetical scenario in which the exposure(s) are reduced by a certain amount given as
 where XC is the contrast matrix obtained by subtracting the observed exposure(s) from the hypothetical. The sum of all predicted probabilities for the cases in the sample corresponds to the expected number of cases expected under the hypothetical scenario. The PAF is then calculated as
where XC is the contrast matrix obtained by subtracting the observed exposure(s) from the hypothetical. The sum of all predicted probabilities for the cases in the sample corresponds to the expected number of cases expected under the hypothetical scenario. The PAF is then calculated as where γ is a shrinkage factor defined as
where γ is a shrinkage factor defined as where p is the total degrees of freedom and model χ2 is the likelihood ratio statistic for testing the joint influence of all variables in the model.12 As there were few cases of diabetes relative to the model degrees of freedom, the models may overfit. The shrinkage factor shrinks the PAF towards zero and was used to compensate for the overfitting when generalising the results to the European population.
where p is the total degrees of freedom and model χ2 is the likelihood ratio statistic for testing the joint influence of all variables in the model.12 As there were few cases of diabetes relative to the model degrees of freedom, the models may overfit. The shrinkage factor shrinks the PAF towards zero and was used to compensate for the overfitting when generalising the results to the European population.
Five models were developed with the difference between the models being the hypothetical scenarios. The first scenario assumed a simultaneous 25% decrease in all four contaminants while the remaining scenarios assumed a 25% decrease in a single contaminant while keeping the other contaminants constant. The bootstrap was used to construct 95% bias corrected and accelerated CIs for PAF using 10 000 replicates.13 All analyses were made using R V.3.2.4 (R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://http://www.R-project.org/ 2016).
Burden of disease and economic estimation
The number of diabetes cases among 70–75 years old was estimated by multiplying age-standardised prevalence of diabetes in Europe (6.5%)14 against the population estimate of 70–75 years old.15 Annual cost estimates for diabetes per adult in 2010 were obtained from the analyses by Zhang et al,16 in US dollars. Given that prevalent diabetes results in costs over multiple years of each subsequent lifetime, annual costs were aggregated over a 10-year period, using 3% discounting.
Human participants
PIVUS was approved by the Ethics Committee of the University of Uppsala and the participants gave informed consent. LT signed a New York University School of Medicine Institutional Review Board attestation form documenting the nature of his involvement as non-human participants research.
Results
Table 1 presents descriptive biomarker data analysed in relation to diabetes, documenting exposures similar to those identified in other European populations. All four exposures taken singly had near-significant prevalence ratios (table 2), though reduction of all four exposures by 25% was associated with a 13% (95% CI 2% to 22%) lower prevalence of diabetes, compared with a 40% reduction in diabetes prevalence in association with an identical (25%) reduction in BMI. Extrapolation to Europe suggests that 152 481 cases of diabetes could be prevented by reducing EDC exposure, compared with 469 172 produced by a population-wide 25% reduction in BMI (table 3). Economic benefits of reducing EDC-attributable diabetes were estimated to be €4.51 billion/year compared with the €13.9 billion benefits of reducing BMI.
Biomarkers of exposure in the Prospective Investigation of the Vasculature in Uppsala Seniors
Population attributable risk in multiexposure models
Attributable disease and cost estimates
Discussion
The present study confirms substantial contribution, especially of mixtures of EDCs, to adult type 2 diabetes, and large annual costs of medical care.4 While some will question extrapolation on limited data, our findings regarding chemical diabetogens are not unique and have also been found by several other research groups.5 ,17–22 These epidemiological findings are likely to be causal, since they are in line with experimental mechanistic data.23–32
All the same, we acknowledge that residual confounding may have resulted in effect overestimation for the chemical exposures studied. The calculated PAFs may not apply to older age ranges insofar as biomarker levels have decreased ecologically. Exposures much earlier than study entry may have contributed to those measured in biomarkers at study entry.
It should be emphasised that PCBs have already been banned, under the Stockholm Convention.33 ,34 The pesticide dichlorodiphenyltrichloroethylene, for which the measured levels of p,p′-DDE are proxy, has also been banned, except for the eradication of malaria in some parts of southern Africa. Long-chain perfluoroalkyl compounds, including PFNA, have completed a voluntary phase-out in the USA, though the expected reductions in serum PFNA have not been identified.35 ,36
Yet, healthcare providers can advise patients to reduce their exposure to diabetogenic contaminants identified in this study. Choosing personal care products labelled as ‘phthalate free’ has reduced urinary levels of MEP by 27% in young girls in one study.37 Other phthalates known to be metabolic disruptors are known food contaminants, and a fresh food intervention has produced even larger reductions in exposure.38 Short-chain PFASs contaminate food through packaging and contact surfaces, and are increasingly found in food.39 Consumption of a diet according to WHO recommendations has been associated with lower levels of PFASs and PCBs.40 ,41
Our findings also speak the need for a strong regulatory framework that proactively identifies chemical hazards before they are widely used, and the use of safer alternatives. The European Union is actively considering regulations to limit such exposures,4 and the USA recently revised the Toxic Substances Control Act,42 but does not consider endocrine disruption. In the absence of such a framework, newly developed synthetic chemicals may emerge as diabetogenic exposures, replacing banned or substituted hazards as contributors.
Conclusions
Environmental contaminants contribute substantially to diabetes in the elderly, with costs in Europe likely to be in billions of Euros.
What is already known on this subject
Increasing evidence suggests that synthetic chemicals commonly found in the environment contribute to metabolic disorders, especially obesity and diabetes. Yet, only one study has quantified attributable disease and costs, did not examine mixtures of chemicals which may have synergistic effects and was limited to effects in middle-aged women.
What this study adds
Reduction of chemical exposures was associated with a 13% (95% CI 2% to 22%) reduction in diabetes in the elderly, preventing 152 481 cases of diabetes in Europe and €4.51 billion/year in associated costs. These findings support regulatory and individual efforts to reduce chemical exposures.
References
Supplementary materials
Press release
Files in this Data Supplement:
Footnotes
Twitter Follow Leonardo Trasande at @leotrasande
Contributors LT wrote the manuscript, and performed economic analyses. PML and LL provided primary study access and reviewed/edited the manuscript. EL performed statistical analyses. LT and PML are the joint guarantors of this work, including the study design, access to data, and the decision to submit and publish the manuscript.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.


