Abstract
Worldwide, an estimated 130–170 million people have HCV infection. HCV prevalence is highest in Egypt at >10% of the general population and China has the most people with HCV (29.8 million). Differences in past HCV incidence and current HCV prevalence, together with the generally protracted nature of HCV disease progression, has led to considerable diversity in the burden of advanced liver disease in different countries. Countries with a high incidence of HCV or peak incidence in the recent past will have further escalations in HCV-related cirrhosis and hepatocellular carcinoma (HCC) over the next two decades. Acute HCV infection is difficult to detect because of the generally asymptomatic nature of the disease and the marginalization of at-risk populations. Around 25% of patients with acute HCV infection undergo spontaneous clearance, with increased rates among those with favourable IL28B genotypes, acute symptoms and in women. The remaining 75% of patients progress to chronic HCV infection and are subsequently at risk of progression to hepatic fibrosis, cirrhosis and HCC. Chronic hepatitis C generally progresses slowly in the initial two decades, but can be accelerated during this time as a result of advancing age and co-factors such as heavy alcohol intake and HIV co-infection.
Key Points
-
Although many countries in Asia have a low-to-intermediate prevalence of HCV, around half of the global population of patients infected with HCV reside in this region
-
Many countries have 'ageing cohorts' of people with HCV owing to peak HCV incidences in the recent past (2000 in Australia, 1980s in the USA) or distant past (1920–1940s in Japan)
-
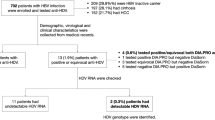
Changes in levels of HCV RNA during acute HCV infection might guide early therapeutic intervention, with levels in patients with viral clearance or persistence diverging after 3–4 months of infection
-
The disease progression of chronic HCV infection often accelerates after 20 years of infection, with lifestyle factors key drivers of hepatic fibrosis
-
Direct-acting antiviral therapies should provide a paradigm shift in treatment over the next few years
-
However, unless rates of diagnosis of HCV and access to treatment improve dramatically, direct-acting antivirals will have a limited effect on global disease burden
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Grebely, J. & Dore, G. J. What is killing people with hepatitis C virus infection? Semin. Liver Dis. 31, 331–339 (2011).
Dore, G. J. The changing therapeutic landscape for hepatitis C. Med. J. Aust. 196, 629–632 (2012).
Thomas, D. L. Curing hepatitis C with pills: A step toward global control. Lancet 376, 1441–1442 (2010).
Lavanchy, D. The global burden of hepatitis C. Liver Int. 29, 74–81 (2009).
The Global Burden of Hepatitis C Working Group. Global burden of disease (GBD) for hepatitis C. J. Clin. Pharmacol. 44, 20–29 (2004).
Lavanchy, D. Evolving epidemiology of hepatitis C virus. Clin. Microbiol. Infect. 17, 107–115 (2011).
World Health Organization. Hepatitis C–global prevalence (update). Weekly Epidemiological Record 49, 425–427 (1999).
Nerrienet, E. et al. Hepatitis C virus infection in Cameroon: A cohort-effect. J. Med. Virol. 76, 208–214 (2005).
Guerra, J., Garenne, M., Mohamed, M. K. & Fontanet, A. HCV burden of infection in Egypt: results from a nationwide survey. J. Viral Hepat. 19, 560–567 (2012).
Centers for Disease Control and Prevention. Viral hepatitis surveillance—United States, 2010 (updated 2012) [online], (2012).
Rantala, M. & van de Laar, M. J. Surveillance and epidemiology of hepatitis B and C in Europe—a review. Euro Surveill. 13, 194–204 (2008).
Armstrong, G. L. et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann. Intern. Med. 144, 705–714 (2006).
Vriend, H. J. et al. Hepatitis C virus seroprevalence in the Netherlands. Eur. J. Pub. Health 22, 819–821 (2012).
Dalgard, O., Jeansson, S., Skaug, K., Raknerud, N. & Bell, H. Hepatitis C in the general adult population of Oslo: prevalence and clinical spectrum. Scand. J. Gastroenterol. 38, 864–870 (2003).
Harris, R. J. et al. Hepatitis C prevalence in England remains low and varies by ethnicity: An updated evidence synthesis. Eur. J. Pub. Health 22, 187–192 (2012).
Meffre, C. et al. Prevalence of hepatitis B and hepatitis C virus infections in France in 2004: Social factors are important predictors after adjusting for known risk factors. J. Med. Virol. 82, 546–555 (2010).
The Kirby Institute. HIV, Viral Hepatitis and Sexually Transmissible Infections in Australia. Annual Surveillance Report 2012 (The Kirby Institute, the University of New South Wales, 2012).
Tanaka, J. et al. Sex- and age-specific carriers of hepatitis B and C viruses in Japan estimated by the prevalence in the 3,485,648 first-time blood donors during 1995–2000. Intervirology 47, 32–40 (2004).
Alter, M. J. Epidemiology of hepatitis C virus infection. World J. Gastroenterol. 13, 2436–2441 (2007).
Alter, M. J. et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N. Engl. J. Med. 341, 556–562 (1999).
Chak, E., Talal, A. H., Sherman, K. E., Schiff, E. R. & Saab, S. Hepatitis C virus infection in USA: An estimate of true prevalence. Liver Int. 31, 1090–1101 (2011).
Armstrong, G. L., Alter, M. J., McQuillan, G. M. & Margolis, H. S. The past incidence of hepatitis C virus infection: Implications for the future burden of chronic liver disease in the United States. Hepatology 31, 777–782 (2000).
Williams, I. Epidemiology of hepatitis C in the United States. Am. J. Med. 107, 2–9 (1999).
Williams, I. T., Bell, B. P., Kuhnert, W. & Alter, M. J. Incidence and transmission patterns of acute hepatitis C in the United States, 1982–2006. Arch. Intern. Med. 171, 242–248 (2011).
Razali, K. et al. Modelling the hepatitis C virus epidemic in Australia. Drug Alcohol Depend. 91, 228–235 (2007).
Sievert, W. et al. A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int. 31, 61–80 (2011).
Yoshizawa, H. Hepatocellular carcinoma associated with hepatitis C virus infection in Japan: Projection to other countries in the foreseeable future. Oncology 62, 8–17 (2002).
Tanaka, Y. et al. Molecular evolutionary analyses implicate injection treatment for schistosomiasis in the initial hepatitis C epidemics in Japan. J. Hepatol. 42, 47–53 (2005).
Mühlberger, N. et al. HCV-related burden of disease in Europe: A systematic assessment of incidence, prevalence, morbidity, and mortality. BMC Public Health 9, 34 (2009).
Gheorghe, L., Iacob, S. & Csiki, I. E. Prevalence of hepatitis C in Romania: Different from European rates? J. Hepatol. 49, 661–662 (2008).
Lvov, D. K. et al. Prevalence of hepatitis C virus and distribution of its genotypes in Northern Eurasia. Arch. Virol. 141, 1613–1622 (1996).
Ansaldi, F. et al. Different seroprevalence and molecular epidemiology patterns of hepatitis C virus infection in Italy. J. Med. Virol. 76, 327–332 (2005).
Delarocque-Astagneau, E. et al. An incident case-control study of modes of hepatitis C virus transmission in France. Ann. Epidemiol. 17, 755–762 (2007).
Duberg, A., Janzon, R., Bäck, E., Ekdahl, K. & Blaxhult, A. The epidemiology of hepatitis C virus infection in Sweden. Euro Surveill. 13, 181–185 (2008).
Prasad, L. et al. Cohort profile: The Swiss Hepatitis C Cohort Study (SCCS). Int. J. Epidemiol. 36, 731–737 (2007).
Cornberg, M. et al. A systematic review of hepatitis C virus epidemiology in Europe, Canada and Israel. Liver Int. 31, 30–60 (2011).
Defossez, G. et al. Evaluation of the French national plan to promote screening and early management of viral hepatitis C, between 1997 and 2003: A comparative cross-sectional study in Poitou-Charentes region. Eur. J. Gastroenterol. Hepatol. 20, 367–372 (2008).
Delarocque-Astagneau, E. et al. The impact of the prevention programme of hepatitis C over more than a decade: The French experience. J. Viral Hepat. 17, 435–443 (2010).
Naoumov, N. V. Hepatitis C virus infection in Eastern Europe. J. Hepatol. 31 (Suppl. 1), 84–87 (1999).
Esteban, J. I., Sauleda, S. & Quer, J. The changing epidemiology of hepatitis C virus infection in Europe. J. Hepatol. 48, 148–162 (2008).
Miller, F. D. & Abu-Raddad, L. J. Evidence of intense ongoing endemic transmission of hepatitis C virus in Egypt. Proc. Natl Acad. Sci. 107, 14757–14762 (2010).
Centers for Disease Control and Prevention. Progress toward prevention and control of hepatitis C virus infection—Egypt, 2001–2012. MMWR 61, 545–549 (2012).
Frank, C. et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet 355, 887–891 (2000).
Paez Jimenez, A. et al. HCV iatrogenic and intrafamilial transmission in Greater Cairo, Egypt. Gut 59, 1554–1560 (2010).
Plancoulaine, S. et al. Dissection of familial correlations in hepatitis C virus (HCV) seroprevalence suggests intrafamilial viral transmission and genetic predisposition to infection. Gut 57, 1268–1274 (2008).
Mohamed, M. K. et al. Intrafamilial transmission of hepatitis C in Egypt. Hepatology 42, 683–687 (2005).
Ali, S. A., Donahue, R. M. J., Qureshi, H. & Vermund, S. H. Hepatitis B and hepatitis C in Pakistan: prevalence and risk factors. Int. J. Infect. Dis. 13, 9–19 (2009).
Waheed, Y., Shafi, T., Safi, S. Z. & Qadri, I. Hepatitis C virus in Pakistan: A systematic review of prevalence, genotypes and risk factors. World J. Gastroenterol. 15, 5647–5653 (2009).
Merat, S. et al. Seroprevalence of hepatitis C virus: The first population-based study from Iran. Int. J. Infect. Dis. 14, e113–e116 (2010).
Alavian, S. M. Hepatitis C infection in Iran; A review article. Iranian J. Clin. Infect. Dis. 4, 47–59 (2009).
Xia, G.-L. et al. Prevalence of hepatitis B and C virus infections in the general Chinese population. Results from a nationwide cross-sectional seroepidemiologic study of hepatitis A, B, C, D, and E virus infections in China, 1992. Int. Hepatol. Comm. 5, 62–73 (1996).
Lu, J. et al. General epidemiological parameters of viral hepatitis A, B, C, and E in six regions of China: a cross-sectional study in 2007. PLoS ONE 4, e8467 (2009).
Liu, F. et al. Hepatitis C seroprevalence and associated risk factors, Anyang, China. Emerg. Infect. Dis. 15, 1819–1822 (2009).
Yan, Z. et al. Changing pattern of clinical epidemiology on hepatitis C virus infection in Southwest China. Hepatitis Monthly 12, 196–204 (2012).
Paez Jimenez, A. et al. Injection drug use is a risk factor for HCV infection in urban Egypt. PLoS ONE 4, e7193 (2009).
Thaikruea, L. et al. Risk factors for hepatitis C virus infection among blood donors in northern Thailand. Transfusion 44, 1433–1440 (2004).
Ohno, T. et al. New hepatitis C virus (HCV) genotyping system that allows for identification of HCV genotypes 1a, 1b, 2a, 2b, 3a, 3b, 4, 5a, and 6a. J. Clin. Microbiol. 35, 201–207 (1997).
Simmonds, P. et al. Geographical distribution of hepatitis C virus genotypes in blood donors: An international collaborative survey. J. Clin. Microbiol. 32, 884–892 (1994).
Kaba, S. et al. Molecular epidemiology of hepatitis C in Australia. J. Gastroenterol. Hepatol. 13, 914–920 (1998).
Chlabicz, S. et al. Changing HCV genotypes distribution in Poland—Relation to source and time of infection. J. Clin. Virol. 42, 156–159 (2008).
Tallo, T. et al. Genetic characterization of hepatitis C virus strains in Estonia: Fluctuations in the predominating subtype with time. J. Med. Virol. 79, 374–382 (2007).
Katsoulidou, A. et al. Molecular epidemiology of hepatitis C virus (HCV) in Greece: Temporal trends in HCV genotype-specific incidence and molecular characterization of genotype 4 isolates. J. Viral Hepat. 13, 19–27 (2006).
Payan, C. et al. Changing of hepatitis C virus genotype patterns in France at the beginning of the third millenium: The GEMHEP GenoCII Study. J. Viral Hepat. 12, 405–413 (2005).
Delwaide, J. et al. HCV genotype 4 in Belgium: three distinct patterns among patients from European and African origin. Eur. J. Gastroenterol. Hepatol. 18, 707–712 (2006).
Fernández-Arcás, N. et al. High prevalence of hepatitis C virus subtypes 4c and 4d in Malaga (Spain): Phylogenetic and epidemiological analyses. J. Med. Virol. 78, 1429–1435 (2006).
Kamal, S. M. & Nasser, I. A. Hepatitis C genotype 4: What we know and what we don't yet know. Hepatology 47, 1371–1383 (2008).
Shobokshi, O. A., Serebour, F. E., Skakni, L., Al-Saffy, Y. H. & Ahdal, M. N. Hepatitis C genotypes and subtypes in Saudi Arabia. J. Med. Virol. 58, 44–48 (1999).
Antaki, N. et al. The unexpected discovery of a focus of hepatitis C virus genotype 5 in a Syrian province. Epidemiol. Infect. 137, 79–84 (2009).
Murphy, D. G. et al. Biological and clinicopathological features associated with hepatitis C virus type 5 infections. J. Hepatol. 24, 109–113 (1996).
Duc, A. P. et al. High prevalence of hepatitis C virus genotype 6 in Vietnam. Asian Pac. J. Allergy Immunol. 27, 153–160 (2009).
Li, C. S. Y., Chan, P. K. S. & Tang, J. W. Molecular epidemiology of hepatitis C genotype 6a from patients with chronic hepatitis C from Hong Kong. J. Med. Virol. 81, 628–633 (2009).
Lozano, R. et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2095–2128 (2012).
Perz, J. F., Armstrong, G. L., Farrington, L. A., Hutin, Y. J. F. & Bell, B. P. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J. Hepatol. 45, 529–538 (2006).
Wasley, A. & Alter, M. J. Epidemiology of hepatitis C: Geographic differences and temporal trends. Semin. Liver Dis. 20, 1–16 (2000).
Anwar, W. A., Khaled, H. M., Amra, H. A., El-Nezami, H. & Loffredo, C. A. Changing pattern of hepatocellular carcinoma (HCC) and its risk factors in Egypt: Possibilities for prevention. Mutat. Res. 659, 176–184 (2008).
Deuffic-Burban, S., Mohamed, M. K., Larouze, B., Carrat, F. & Valleron, A.-J. Expected increase in hepatitis C-related mortality in Egypt due to pre-2000 infections. J. Hepatol. 44, 455–461 (2006).
Arfè, A. et al. Cancer mortality trend analysis in Italy, 1970–2007. Eur. J. Cancer Prevent. 20, 364–374 (2011).
Tanaka, H. et al. Declining incidence of hepatocellular carcinoma in Osaka, Japan, from 1990 to 2003. Ann. Intern. Med. 148, 820–826 (2008).
Davis, G. L., Alter, M. J., El-Serag, H., Poynard, T. & Jennings, L. W. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology 138, 513–521 (2010).
Amin, J. et al. Liver cancer and hepatitis B and C in New South Wales, 1990–2002: A linkage study. Aust. NZ J. Public Health 31, 475–482 (2007).
Deuffic-Burban, S., Mathurin, P. & Valleron, A. J. Modelling the past, current and future HCV burden in France: Detailed analysis and perspectives. Stat. Methods Med. Res. 18, 233–252 (2009).
Sagmeister, M., Renner, E. L., Mullhaupt, B. & Wong, J. B. Simulation of hepatitis C based on a mandatory reporting system. Eur. J. Gastroenterol. Hepatol. 14, 25–34 (2002).
Orland, J. R., Wright, T. L. & Cooper, S. Acute hepatitis C. Hepatology 33, 321–327 (2001).
Cox, A. L. et al. Prospective evaluation of community-acquired acute-phase hepatitis C virus infection. Clin. Infect. Dis. 40, 951–958 (2005).
Page-Shafer, K. et al. Testing strategy to identify cases of acute hepatitis C virus (HCV) infection and to project HCV incidence rates. J. Clin. Microbiol. 46, 499–506 (2008).
Glynn, S. A. et al. Dynamics of viremia in early hepatitis C virus infection. Transfusion 45, 994–1002 (2005).
Hajarizadeh, B., Grebely, J. & Dore, G. J. Case definitions for acute hepatitis C virus infection: A systematic review. J. Hepatol. 57, 1349–1360 (2012).
Liu, L., Fisher, B. E., Thomas, D. L., Cox, A. L. & Ray, S. C. Spontaneous clearance of primary acute hepatitis C virus infection correlated with high initial viral RNA level and rapid HVR1 evolution. Hepatology 55, 1684–1691 (2012).
Hajarizadeh, B. et al. in The 8th Australasian Viral Hepatitis Conference 150 (Australasian Society for HIV Medicine, Auckland, New Zealand, 2012).
McGovern, B. H. et al. Improving the diagnosis of acute hepatitis C virus infection with expanded viral load criteria. Clin. Infect. Dis. 49, 1051–1060 (2009).
Grebely, J. et al. Plasma interferon-γ-inducible protein-10 (IP-10) levels during acute hepatitis C virus infection. Hepatology 57, 2124–2134 (2013).
Mosley, J. W. et al. Viral and host factors in early hepatitis C virus infection. Hepatology 42, 86–92 (2005).
Micallef, J. M., Kaldor, J. M. & Dore, G. J. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J. Viral Hepat. 13, 34–41 (2006).
Page, K. et al. Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incident infection, resolution, and reinfection. J. Infect. Dis. 200, 1216–1226 (2009).
Lemon, S. M. Induction and evasion of innate antiviral responses by hepatitis C virus. J. Biol. Chem. 285, 22741–22747 (2010).
Rehermann, B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J. Clin. Invest. 119, 1745–1754 (2009).
Takaki, A. et al. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat. Med. 6, 578–582 (2000).
Tillmann, H. L. et al. A polymorphism near IL28B is associated with spontaneous clearance of acute hepatitis C virus and jaundice. Gastroenterology 139, 1586–1592 (2010).
Grebely, J. et al. Potential role for interleukin-28B genotype in treatment decision-making in recent hepatitis C virus infection. Hepatology 52, 1216–1224 (2010).
Thomas, D. L. et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 461, 798–801 (2009).
Marcello, T. et al. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology 131, 1887–1898 (2006).
Ray, S. C. et al. Acute hepatitis C virus structural gene sequences as predictors of persistent viremia: hypervariable region 1 as a decoy. J. Virol. 73, 2938–2946 (1999).
Harris, H. E. et al. Does the clinical outcome of hepatitis C infection vary with the infecting hepatitis C virus type? J. Viral Hepat. 14, 213–220 (2007).
Poynard, T., Bedossa, P. & Opolon, P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet 349, 825–832 (1997).
Wiley, T. E., Brown, J. & Chan, J. Hepatitis C infection in African Americans: Its natural history and histological progression. Am. J. Gastroenterol. 97, 700–706 (2002).
Minola, E. et al. Age at infection affects the long-term outcome of transfusion-associated chronic hepatitis C. Blood 99, 4588–4591 (2002).
Graham, C. S. et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: A meta-analysis. Clin. Infect. Dis. 33, 562–569 (2001).
Gaeta, G. B. et al. Epidemiological and clinical burden of chronic hepatitis B virus/hepatitis C virus infection. A multicenter Italian study. J. Hepatol. 39, 1036–1041 (2003).
Ratziu, V., Munteanu, M., Charlotte, F., Bonyhay, L. & Poynard, T. Fibrogenic impact of high serum glucose in chronic hepatitis C. J. Hepatol. 39, 1049–1055 (2003).
Hui, J. M. et al. Insulin resistance is associated with chronic hepatitis C and virus infection fibrosis progression. Gastroenterology 125, 1695–1704 (2003).
Hourigan, L. F. et al. Fibrosis in chronic hepatitis C correlates significantly with body mass index and steatosis. Hepatology 29, 1215–1219 (1999).
Leandro, G. et al. relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology 130, 1636–1642 (2006).
Hutchinson, S. J., Bird, S. M. & Goldberg, D. J. Influence of alcohol on the progression of hepatitis C virus infection: A meta-analysis. Clin. Gastroenterol. Hepatol. 3, 1150–1159 (2005).
Hézode, C. et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology 42, 63–71 (2005).
Hézode, C. et al. Impact of smoking on histological liver lesions in chronic hepatitis C. Gut 52, 126–129 (2003).
Pessione, F. et al. Cigarette smoking and hepatic lesions in patients with chronic hepatitis C. Hepatology 34, 121–125 (2001).
Bochud, P. Y. et al. Genotype 3 is associated with accelerated fibrosis progression in chronic hepatitis C. J. Hepatol. 51, 655–666 (2009).
Poynard, T. et al. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis C. J. Hepatol. 34, 730–739 (2001).
Seeff, L. B. Natural history of chronic hepatitis C. Hepatology 36, s35–s46 (2002).
Thein, H. H., Yi, Q., Dore, G. J. & Krahn, M. D. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: A meta-analysis and meta-regression. Hepatology 48, 418–431 (2008).
Thein, H. H., Yi, Q., Dore, G. J. & Krahn, M. D. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS 22, 1979–1991 (2008).
Freeman, A. J. et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology 34, 809–816 (2001).
El-Serag, H. B. & Rudolph, K. L. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132, 2557–2576 (2007).
Fattovich, G., Stroffolini, T., Zagni, I. & Donato, F. Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology 127, S35–S50 (2004).
Hamada, H. et al. Impact of aging on the development of hepatocellular carcinoma in patients with posttransfusion chronic hepatitis C. Cancer 95, 331–339 (2002).
Masuzaki, R. et al. Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology 49, 1954–1961 (2009).
McDonald, S. A. et al. A population-based record linkage study of mortality in hepatitis C-diagnosed persons with or without HIV coinfection in Scotland. Stat. Methods Med. Res. 18, 271–283 (2009).
Walter, S. R. et al. Trends in mortality after diagnosis of hepatitis B or C infection: 1992–2006. J. Hepatol. 54, 879–886 (2011).
Grebely, J. et al. Impact of hepatitis C virus infection on all-cause and liver-related mortality in a large community-based cohort of inner city residents. J. Viral Hepat. 18, 32–41 (2011).
Degenhardt, L. et al. Mortality among clients of a state-wide opioid pharmacotherapy program over 20 years: Risk factors and lives saved. Drug Alcohol Depend. 105, 9–15 (2009).
Lee, M. H. et al. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J. Infect. Dis. 206, 469–477 (2012).
Giordano, T. P. et al. Risk of non-Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. JAMA 297, 2010–2017 (2007).
Bonner, J., Barritt, A. S., Fried, M. & Evon, D. Time to rethink antiviral treatment for hepatitis C in patients with coexisting mental health/substance abuse issues. Dig. Dis. Sci. 57, 1469–1474 (2012).
Arora, S. et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N. Engl. J. Med. 364, 2199–2207 (2011).
Larrey, D. et al. Education by a nurse increases response of patients with chronic hepatitis C to therapy with peginterferon-α2a and ribavirin. Clin. Gastroenterol. Hepatol. 9, 781–785 (2011).
Grebely, J. et al. Directly observed therapy for the treatment of hepatitis C virus infection in current and former injection drug users. J. Gastroenterol. Hepatol. 22, 1519–1525 (2007).
Grebely, J. et al. Optimizing assessment and treatment for hepatitis C virus infection in illicit drug users: A novel model incorporating multidisciplinary care and peer support. Eur. J. Gastroenterol. Hepatol. 22, 270–277 (2010).
Norman, J. et al. The acceptability and feasibility of peer worker support role in community based HCV treatment for injecting drug users. Harm. Reduction J. 5, 8 (2008).
Sylvestre, D. L. & Zweben, J. E. Integrating HCV services for drug users: A model to improve engagement and outcomes. Int. J. Drug Policy 18, 406–410 (2007).
Butt, A. A. et al. Rate and predictors of treatment prescription for hepatitis C. Gut 56, 385–389 (2007).
Lettmeier, B. et al. Market uptake of new antiviral drugs for the treatment of hepatitis C. J. Hepatol. 49, 528–536 (2008).
Volk, M. L., Tocco, R., Saini, S. & Lok, A. S. F. Public health impact of antiviral therapy for hepatitis C in the United States. Hepatology 50, 1750–1755 (2009).
Acknowledgements
The Kirby Institute is funded by the Australian Government Department of Health and Ageing and is affiliated with the Faculty of Medicine, The University of New South Wales. B. Hajarizadeh is an Australian Postgraduate Award PhD scholar. J. Grebely is supported by a National Health and Medical Research Council Career Development Fellowship. G. J. Dore is supported by a National Health and Medical Research Council Practitioner Research Fellowship. The authors would also like to thank Dr Homie Razavi (Center for Disease Analysis) for contributing epidemiological data for the figures included in this manuscript.
Author information
Authors and Affiliations
Contributions
B. Hajarizadeh researched data for the article, contributed to discussion of content, wrote the article and reviewed/edited the manuscript before submission. J. Grebely and G. J. Dore researched data for the article, contributed to discussion of content and reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J. Grebely is a consultant/advisor for Merck. G. J. Dore is a consultant/advisor and has received research grants from AbbeVie, Bristol Myers Squibb, Gilead, Janssen, Merck and Roche. B. Hajarizadeh declares no competing interests.
Rights and permissions
About this article
Cite this article
Hajarizadeh, B., Grebely, J. & Dore, G. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol 10, 553–562 (2013). https://doi.org/10.1038/nrgastro.2013.107
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2013.107
This article is cited by
-
Long-term trends in incidence, mortality and burden of liver cancer due to specific etiologies in Hubei Province
Scientific Reports (2024)
-
Impact of Hepatitis B on Complications and Functional Outcomes in Patients Undergoing TKR
Indian Journal of Orthopaedics (2024)
-
Need for integration of hepatitis C (HCV) services in community-based settings for people who inject drugs: results from a global values and preferences survey
Harm Reduction Journal (2023)
-
Toll-like receptor 7 and RIG-I-like receptors expression in peripheral blood mononuclear cells of naïve patients with hepatitis C
BMC Research Notes (2023)
-
Trend analysis of hepatitis B and C among patients visiting health facility of Tigrai, Ethiopia, 2014–2019
BMC Gastroenterology (2023)