Abstract
Air quality health impact assessment (HIA) synthesizes information about air pollution exposures, health effects, and population vulnerability for regulatory decision-making and public engagement. HIAs often use annual average county or regional data to estimate health outcome incidence rates that vary substantially by season and at the subcounty level. Using New York City as an example, we assessed the sensitivity of estimated citywide morbidity and mortality attributable to ambient fine particulate matter (PM2.5) and ozone to the geographic (county vs. neighborhood) and temporal (seasonal vs. annual average) resolution of health incidence data. We also used the neighborhood-level analysis to assess variation in estimated air pollution impacts by neighborhood poverty concentration. Estimated citywide health impacts attributable to PM2.5 and ozone were relatively insensitive to the geographic resolution of health incidence data. However, the neighborhood-level analysis demonstrated increasing impacts with greater neighborhood poverty levels, particularly for PM2.5-attributable asthma emergency department visits, which were 4.5 times greater in high compared to low-poverty neighborhoods. PM2.5-attributable health impacts were similar using seasonal and annual average incidence rates. Citywide ozone-attributable asthma morbidity was estimated to be 15 % lower when calculated from seasonal, compared to annual average incidence rates, as asthma morbidity rates are lower during the summer ozone season than the annual average rate. Within the ozone season, 57 % of estimated ozone-attributable emergency department for asthma in children occurred in the April–June period when average baseline incidence rates are higher than in the July–September period when ozone concentrations are higher. These analyses underscore the importance of utilizing spatially and temporally resolved data in local air quality impact assessments to characterize the overall city burden and identify areas of high vulnerability.
Similar content being viewed by others
Introduction
Fine particulate matter (PM2.5) and ozone (O3) are common combustion-related pollutants that contribute to increased emergency department visits, hospitalizations, and deaths due to respiratory and cardiovascular disease (US EPA 2006a, 2009). While these effects are documented in a large number of peer-reviewed published studies, it is challenging to distill this extensive evidence to describe the public health burden attributable to air pollution to convey the importance of emissions reductions initiatives to elected officials and the general public.
Air quality health impact assessments (HIA) are one approach to synthesizing information about air pollution exposures, health effects, and population vulnerability. Analysis methods have become relatively standardized and used by the US Environmental Protection Agency (US EPA 2008, 2010a) and state and regional groups (NESCAUM 2008) to estimate potential health benefits of air quality regulations. These methods have also been applied in estimating the global and national public health burden of pollutant exposures attributable to current ambient concentrations relative to some estimate of background concentration (Cohen et al. 2005; US EPA 2010a; Fann et al. 2011a, b).
In recent years, there has been growing recognition of the potential value for stakeholder engagement of applying national regulatory HIA methods at a local scale by using health and pollutant exposure data that are more spatially resolved than the county or 12-km model grid cell level typically available and used in air pollution HIA (US EPA 2008, 2010a; Fann et al. 2011a, b; Hubbell et al. 2005; Matte et al. 2009). The use of more finely spatially resolved exposure and health data is essential to support transition from the currently mandated monitor-based National Ambient Air Quality Standards (NAAQS) attainment approach to multipollutant, risk-based air quality management approaches (National Research Council 2004; Dominici et al. 2010). Furthermore, accounting for neighborhood level variation in exposures and morbidity and mortality rates allows for assessment of disparities in air pollution impacts. However, significant methodological challenges need to be addressed when conducting health impact assessments at a local scale, and approaches have been proposed for concentration–response (C–R) function selection, exposure estimation, and baseline incidence data choices (Hubbell et al. 2009; Fuentes 2009).
Seasonal variation in both air pollutants and rates of morbidity and mortality also complicate health impact assessment. For example, asthma hospitalization rates in children typically increase in the early fall and remain higher than summertime rates through much of the spring (Silverman et al. 2005). However, some prior health impact assessments have applied annual average baseline mortality and morbidity rates in calculating health impact estimates associated with changes in air quality that vary by season (Hubbell et al. 2005; US EPA 2008). Recent urban analyses of PM2.5 risk applied seasonal baseline morbidity rates when seasonal effect estimates were available (US EPA 2010a). To our knowledge, however, there has not been an analyses examining how sensitive the estimates of the health impacts of changes in air quality are to accounting for seasonal variability in baseline incidence rates.
Using methods we previously employed to derive estimates of the public health burden attributable to current levels of PM2.5 and O3 in New York City (NYCDOHMH 2011a), we assessed how estimates vary with differing spatial resolution (county vs. neighborhood level) of the analysis and the temporal resolution (annual average vs. seasonal) of baseline health incidence rates. We also quantified disparities in impacts by area-based poverty concentration.
Methods
Overall approach
We calculated the burden of exposure to current levels of ambient PM2.5 and ozone in New York City using previously described methods (Hubbell et al. 2009; Fann et al. 2011a, b). Briefly, we applied evidence from published time-series and cohort studies relating ambient air pollutant concentrations to health outcomes to local data on air pollutant levels, baseline mortality and morbidity rates, and exposed populations. Changes in morbidity and mortality attributed to changes in air pollution were calculated using health impact functions derived from log-linear models relating the risk of disease or death to ambient concentrations of air pollutants of interest:
Where ΔI is the change in the number of health events associated with the change in air pollutant concentration (ΔX), β is the effect coefficient from the epidemiological study, P is the exposed population, and I 0 is the baseline rate of disease or death.
All health impact calculations were conducted using US Environmental Protection Agency’s Benefits Mapping and Analysis Program (BenMAP) Version 4.0, a GIS-based platform that allows analysts to estimate the health impacts associated with user-defined changes in air quality (US EPA 2010b). BenMAP has been used extensively for regulatory applications such as Environmental Protection Agency (EPA)’s analysis of the Federal Transport Rule (US EPA 2011), in evaluation of ozone and PM2.5 National Ambient Air Quality Standards (US EPA 2006b, 2008), and as part of State air quality management planning (NYSDEC 2011).
Current air quality data
Air quality data from EPA’s Air Quality System (AQS) were acquired from all regulatory monitors in the five counties of New York City and the seven adjacent counties in New York State and New Jersey for the 3 years from 2005 to 2007.
PM2.5 data were obtained from 24 monitors collecting integrated 24-h filter-based samples by federal reference methods. Three monitors reported data on a daily schedule while 18 reported on an every third day schedule and three reported on an every sixth day schedule. Daily average values at each monitor were averaged by quarter (Jan 1–March 31, April 1–June 30, July 1–Sep 30, and Oct 1–Dec 31) within each year, and then each quarter was averaged across 3 years. These 3-year quarterly averages were used to characterize baseline air quality while reducing the influence of year-to-year variation due to weather.
Ozone data were obtained for the seven monitors in the region reporting data from 2005 to 2007. Hourly ozone data were used to calculate daily exposure metrics including the daily 8-h maximum, 24-h average, and 4-h afternoon average (1:00–5:00 p.m.). Daily metrics at each monitor were then averaged for each of the two quarters comprising the New York City ozone season (April 1–June 30, July 1–Sep 30) within each year, and then each quarter was averaged across 3 years.
Average concentrations for each quarter were assigned to each of 42 zip code aggregate-based New York City United Hospital Fund (UHF) neighborhoods using an averaging approach within BenMAP known as the Voronoi neighbor averaging (VNA; US EPA 2010b). In short, the VNA algorithm, used in prior air quality HIAs (Hubbell et al. 2005; Fann and Risley 2011), identifies monitors that best surround a point of interest (in this case, the centroid of a given neighborhood or county) then calculates the inverse distance-weighted average concentration of the values from these monitors.
Comparison scenario
We estimated the burden of exposures to current levels of PM2.5 and ozone based on the difference relative to non-anthropogenic, policy-relevant background concentrations (PRB). These background concentrations are derived through atmospheric modeling where all man-made emissions have been removed from the model. For PM2.5, we applied the northeast, season specific, PM2.5 PRB concentrations published in EPA’s 2009 Integrated Science Assessment for Particulate Matter (US EPA 2009) based on modeling performed with the Community Multi-Scale Air Quality Modeling System and the Goddard Earth Observing System (GEOS)-Chem model. Policy-relevant background ranged from 0.67 to 0.87 μg/m3 depending on season, or approximately 5 % of current average PM2.5 concentrations in New York City.
For ozone, we applied PRB estimates modeled by Fiore et al. (2004) using the GEOS-Chem model. In their analysis, Fiore et al. (2004) reported 4-h, afternoon average background ozone estimates during the April to October ozone season for four regions of the USA. We converted the 4-h, afternoon average PRB estimate in the Northeast to a 8-h maximum and 24-h average PRB by computing the ratio of the 4-h average to the 8-h maximum or the 24-h average, calculated from the hourly monitoring data from the sites and time period used in our analysis (Anderson and Bell 2010). Policy-relevant background concentrations were estimated at 21.2 and 20.0 ppb for the 8-h maximum in April–June and July–September, respectively, or approximately 45 % of current average ozone concentrations in New York City and a smaller proportion of the concentration on days with poor air quality.
Selection of concentration–response functions
We reviewed recent epidemiological studies of the relationship of PM2.5 and O3 to mortality, hospital admissions, and emergency department visits and identified those we judged most relevant to the current New York City population for use in the main analyses (Tables 1 and 2). All studies were published in peer-reviewed scientific journals and studies of New York City were used when possible. If local studies were not available, we used recent large, multicity studies or those included in EPA risk analyses (US EPA 2008, 2010a).
Baseline incidence and population data
Mortality data for New York City residents were provided by the New York City Health Department’s Bureau of Vital Statistics for 2005 through 2007. Based on the underlying cause of death, daily counts were summarized and rates of all-cause, cardiovascular, and respiratory mortality were calculated across 22 age and gender groupings (44 total) for each county and 42 UHF neighborhoods. Hospital admissions and emergency room visits data for New York City residents were obtained from the New York Statewide Planning and Research Cooperative System for the same 3-year period (2005–2007). Using diagnostic codes in the hospital discharge data, case definitions were matched to the case definitions used in each of the concentration–response functions used in this analysis. For all mortality and morbidity data, we calculated quarterly rates that matched the quarter definitions in the air quality data and annual average rates, at the UHF and county-level spatial scale. Rates were then averaged over the 3-year period to reduce the influence of random year-to-year variation in rates and to match air quality data.
The 44 age- and sex-specific population estimates for 2005 through 2007 were produced by the New York City Department of Health and Mental Hygiene based on the US Census Bureau Population Estimate Program, supplemented by housing data obtained from the New York City Department of City Planning (NYCDOHMH DES).
To develop estimates of disparity by neighborhood socio-economic status, we stratified the 42 UHF neighborhoods into three poverty tertiles, defined by the percent of neighborhood residents at less than 200 % of the federal poverty threshold, based on data from the 2000 US Census.
Sensitivity analysis
We repeated the citywide pollutant-attributable health burden calculations with varying air quality and baseline health input data to estimate the sensitivity of the final results to method choices. These analyses included:
Sensitivity to spatial scale
We calculated the citywide impact associated with the difference in average air quality levels between the policy relevant background and the 2005–2007 concentrations at the UHF neighborhood level and at the county level. In each case, we matched the baseline incidence rates to the spatial scale of the air quality exposure estimates and summed sub-area impacts to compute estimates of the total citywide burden.
To examine within-city variation in exposure and susceptibility, we computed correlations among air pollutant concentrations, incidence counts, and poverty at the neighborhood level. To assess disparities in air pollution health impacts, UHF neighborhood-level estimates were then aggregated to three neighborhood poverty categories, based on the percent of UHF residents living below 200 % of the federal poverty level grouped by neighborhood tertiles. We then compared the intracity impact gradients and disparities from the neighborhood- and county-scale analyses. For the latter, we estimated neighborhood-level impacts by applying county-level incidence rates and air pollution estimates to all neighborhoods within a county. Gradients were compared using ranges and rank correlations among impact rates computed by the two methods. We also compared the ratios of impact rates in high- compared to low-poverty neighborhoods computed by each method.
Sensitivity to temporal resolution of incidence data
We first used quarterly baseline incidence rates and air quality data to calculate the citywide burden by quarter then summed the results to produce estimates of attributable health events per year. These estimates were compared to those based on annual average health outcome rates and seasonal air quality data.
Results
Air quality and incidence rate estimates
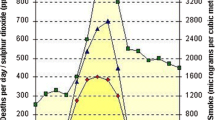
Both PM2.5 and ozone varied seasonally, with PM2.5 levels showing peak values in third (summer) quarter when levels were 17 % higher than the annual average and showing the lowest values in the second (spring) quarter when values were 10 % lower than the annual average (Table 3). Ozone levels also peaked in the third quarter, showing 8 % higher 8-h maximum concentrations than the second quarter.
Interpolation of monitor data to the UHF level provided increased spatial variability in air pollutant concentrations than the county-level interpolation (Fig. 1). For county-level estimates, the ranges of the average concentrations were 17 and 29 % of the mean for PM2.5 and ozone, respectively. The corresponding ranges of the UHF neighborhood level estimated concentrations were 30 and 39 % of the mean concentrations, respectively.
In New York City, there is substantially more spatial variability at the neighborhood than at the county level for baseline incidence rates of all-cause mortality, hospitalizations for cardiovascular and respiratory disease, and emergency department visits for asthma (Fig. 2). Greater neighborhood variability is especially notable for asthma-related emergency department visits where county-level rates vary four-fold, with highest rate in the Bronx, while UHF level rates vary 20-fold with the highest rates found in the neighborhoods of Northern Manhattan followed by the South Bronx. Similarly, hospitalizations for respiratory causes vary by eight-fold across UHF neighborhood, with highest rates in the Bronx and Northern Manhattan while rates only vary by 1.8-fold at the county level. Relatively less spatial variability is seen in mortality and cardiovascular hospitalizations with threefold and 3.2-fold differences across UHF neighborhoods, respectively.
Estimated neighborhood level PM2.5 exposure levels were generally not significantly associated with baseline counts of health events while ozone exposure levels were found to be significantly negatively associated with asthma emergency department visits and hospitalizations (Table 4). Neighborhood poverty was not associated with PM2.5 levels but significantly negatively associated with ozone levels. Conversely, neighborhood poverty level was found to be significantly positively correlated with all health endpoints except mortality.
Sensitivity of overall impact estimates to spatial scale
Citywide health impacts were found to be insensitive to the geographic level used to average air quality and health outcome incidence data. Differences between estimates based on county level compared to neighborhood level analyses were in all cases less than 1 % (Table 5)
Neighborhood disparity by poverty status
For all endpoints except ozone-attributable mortality, higher rates of air pollutant-attributable events are associated with neighborhood poverty (Fig. 3). The largest disparities are estimated for PM2.5 and ozone-attributable asthma emergency department visit rates, which are 4.5 and 3.9 times higher, respectively, in high-poverty neighborhoods, compared to low-poverty neighborhoods. While rates of PM2.5-attributable deaths were 28 % higher in high- compared to low-poverty neighborhoods, the rates of O3-attributable mortality rates were relatively evenly distributed by neighborhood poverty.
The neighborhood-level analysis revealed important subcounty variability in pollutant-attributable health impacts not observed in the county-level analysis. For example, the highest and lowest citywide asthma rates are both found in neighborhoods within New York County (Manhattan): Central Harlem and Greenwich Village/SoHo, respectively. These neighborhoods also fall in the extremes of poverty concentration, with Central Harlem in the high-poverty category and Greenwich Village/SoHo in the low-poverty category.
Sensitivity of the gradient in impact to spatial scale
The range in estimated rates of air pollution attributable events was approximately two to three times greater when applying UHF-level rates compared to applying county-level rates; the neighborhood rankings of attributable impact rates using the two methods were only moderately correlated (Spearman’s rho, 0.41–0.60; Table 6). Comparisons of relative burden in impacts by neighborhood poverty status showed wider disparities when the analysis was conducted using UHF-specific rates as opposed to county-specific rates for all endpoints except ozone-attributable mortality, where limited disparity was observed in either method.
Sensitivity to temporal resolution
Rates of emergency department visits for asthma and respiratory hospitalizations varied significantly by quarter, with the lowest rates occurring in the months of July–September (27 and 21 %, respectively, below the annual average rates) during the peak ozone season (Fig. 4). Mortality and cardiovascular hospitalization rates had less seasonal variation, but were also lower during the July–September period compared to the annual average (by 6 and 3 %, respectively).
Estimated PM2.5-attributable health events based on quarterly incidence rates were generally similar to those based on annual average rates; the largest difference was for cardiovascular hospitalizations which were 7 % lower using seasonal rates (Tables 7 and 8).
In contrast, O3-attributable asthma hospitalizations and emergency department visits estimates were more sensitive to the use of seasonal rates; both were 15 % lower than those calculated using the more conventional approach of applying annual average rates. In addition, seasonally stratified analysis revealed that although average 8-h maximum ozone levels were 8 % higher in the July–September period as compared to the April through June period, an estimated 53 % of all ozone-attributable emergency department visits and 57 % of ozone-attributable emergency department visits among children under 18 years of age occurred in the April–June period.
Discussion
Our analyses found that in New York City, citywide health impact estimates were relatively insensitive to the geographic level (county compared to neighborhood) used to average air quality and health outcome incidence data. However, estimated pollutant-attributable mortality and morbidity varied widely across neighborhoods with differing socio-economic status. Baseline mortality and morbidity rates varied by season, with largest temporal variability found in the rates of emergency department visits for asthma and respiratory hospitalizations. Comparing the use of annual incidence rates versus seasonal rates for a citywide health impact analysis revealed the largest differences in ozone-attributable asthma hospitalizations and emergency department visits because high ozone concentrations occur during times of lower than average baseline incidence rates.
Across all pollutants and health endpoints, we found similar estimates in the burden when calculating at the UHF or county level then aggregating to a citywide estimate. This can be explained by the fact that the change in air quality associated with the rollbacks was relatively poorly correlated geographically with the baseline incidence counts and UHF-level pollutant gradients were not large within individual counties or across the city as a whole. For pollutant/health endpoint combinations where there were limited associations between baseline incidence counts and pollutant levels at the neighborhood (UHF) level, we saw slight differences in the estimates. In other locations where there may be large exposure gradients and strong associations between neighborhoods with high pollution levels and density of susceptible populations, averaging data to a coarser spatial scale for an impact analysis could potentially bias estimates of citywide impacts. Additional factors such as monitor locations and population density will affect exposure assignment and should be considered in developing an appropriate spatial scale for an analysis.
Neighborhood baseline incidence rates serve as one surrogate for relative vulnerability to air pollution health impacts. In New York, a city with very affluent and poor neighborhoods, variation in baseline rates, rather than air quality, account for most of the disparities in pollutant-attributable health where, for example, high-poverty neighborhoods experience 4.5 times higher burden PM2.5-attributable asthma department visits as compared to low-poverty neighborhoods. Although not generally available, relative risks may also vary between neighborhoods based on spatial differences in exposure, co-pollutants, and susceptibility including modification of effect by neighborhood traffic density (Ito et al. 2009). Future improvements in air pollution benefits analyses could come from identification of neighborhood modifiers of air pollution C–R functions and improvements in spatial resolution of air quality monitoring data.
Air quality managers relying on health incidence data available within the BenMAP tool or from publicly accessible sources would typically only able to conduct a county-level analysis. Our analysis of the gradients in pollutant-attributable health impacts found that county-level assessments may not reflect the wider range and greater disparity in impact within-city revealed using neighborhood-scale data.
This level of analysis can be particularly important when evaluating the benefit of control strategies where population susceptibility or air quality improvements may be unevenly distributed within an urban area. For example, in estimating the benefits associated with controls on power plants in the Washington DC area, Levy et al. (2002) reported that an impact assessment that stratified baseline mortality rates and PM2.5 relative risk by population susceptibility did not result in significantly different citywide mortality benefits as compared to unstratified analysis. However, the model that included stratification by education status highlighted the disproportionate impact on less-educated populations. Multipollutant risk-based strategies being developed by EPA have underscored the importance of fine scale, local data in assessing both the magnitude and distribution of benefits associated with air quality improvement strategies, demonstrating that control strategies focused on maximizing benefits in susceptible populations increased overall benefits by almost two-fold while reducing disparities across the population (Fann et al. 2011b). Fine-scale analyses that best reflect neighborhood health conditions are also appropriate in evaluating local initiatives that are aimed at reducing emissions in neighborhoods with high morbidity, such as efforts in New York City that prioritize cleaner fuel boiler conversions in schools in neighborhoods with high asthma rates (NYC 2011).
While environmental justice concerns have traditionally focused on the gradients in air quality exposure, our findings highlight the importance of including gradients in susceptibility, as reflected by baseline morbidity and mortality rates, among groups of differing socioeconomic status (SES). In this analysis, we found no significant gradient in PM2.5 exposures between neighborhoods of differing poverty status, while ozone levels were slightly higher in higher SES communities due to elevated NOx concentrations in areas of Northern Manhattan and the Bronx that increase ozone scavenging (US EPA 2006a). Despite the lack of PM2.5 exposure gradients and negative associations between ozone levels and poverty status, we found wide disparities across SES groups in pollutant-attributable health events due to differences in population susceptibility. Prior US national-level analyses have noted that across the country, the percent poverty of a county was positively associated with PM2.5 levels while an opposite relationship was observed for ozone, with indication that the relationship between county poverty and air quality can vary by geographic region (Miranda et al. 2011). Similarly, European studies have found that while there are not consistent patterns in exposure gradients between SES groups, individuals of low SES were subject to greater health effects of ambient air pollution (Deguen and Zmirou-Navier 2010).
When varying the averaging time of the baseline incidence rates, we found that differences in PM2.5-attributable health impacts were relatively small. The insensitivity of the estimates to temporal resolution of the baseline incidence data is partly due to limited variability in PM2.5 levels across seasons and the fact that seasonal patterns in PM2.5 and health incidents rates were not strongly associated (either positively or negatively).
Conversely, we found that applying an annual incidence rate likely overestimates O3-attributable asthma hospitalizations and emergency department visits and would similarly overestimate the benefits of reducing O3 concentrations. This bias can be explained by the opposing patterns of the baseline rates and ozone concentrations, where peak ozone levels in the third quarter correspond to low baseline rates of asthma-related hospitalizations and emergency department visits. Seasonally stratified analyses also indicated that the majority of ozone-attributable emergency department visits in New York City occur in the earlier portion of the ozone season (April–September).
The seasonality of baseline incidence rates should be considered particularly when the impact of control strategies varies by season. For example, regulatory impact analyses for ozone NAAQS that have applied the readily available annual average baseline incidence rates (US EPA 2008) may overestimate asthma-related impacts due to lower summertime asthma incidence as compared to the annual average. Prior evaluations of ozone-season specific emissions trading strategies such as NOx SIP Call (Burtraw et al. 1998; Environmental Protection Agency 1998) included similar limitations. Air quality advisories for ozone, during which sensitive populations are encouraged to limit exposures and all are encouraged to limit driving, tend to occur later in the ozone season, corresponding to peak ozone concentrations. Our findings suggest that more consideration of springtime ozone impacts is needed in developing air quality management and public health protection strategies. Additionally, health impact assessments of measures to reduce emissions from heating fuels such as those developed in New York City through PlaNYC 2030 (NYC 2011) should account for seasonal variation in emissions and health incidence rates.
A significant limitation in this work and any health impact analysis is uncertainty in underlying data and assumptions, many of which are difficult to quantify. By using local data on neighborhood health events, we have improved upon prior work that assume local rates can be approximated using national, regional, or county data. However, the magnitude of our pollutant-attributable estimates is limited by the uncertainty in the risk estimates derived from the epidemiological literature. For the short-term risk estimates, we have attempted to reduce this uncertainty by applying C–R functions from studies conducted on New York City populations where these estimates have been available, presumably better reflecting underlying susceptibility, local air pollutant mixtures, and PM2.5 composition. However, for endpoints without published epidemiological studies on local populations, we have relied on effect estimates from studies either conducted in other cities or from larger multicity studies, which may result in additional uncertainty in the pollutant-attributable impact estimates (Hubbell et al. 2009). For example, in our analyses, we calculated PM2.5-attributable long-term mortality effects using the Krewski et al. (2009) analysis of the American Cancer Society (ACS). Although this is the largest and most recent study on the effects of PM2.5 on mortality, the ACS population has a smaller proportion of low income and minority participants than the New York City population. Our estimate of the PM2.5 burden would have been more than twice as large had we applied a concentration–response function based on the Laden et al. (2006) analysis of the more diverse Harvard Six Cities cohort (NYCDOHMH 2011a).
An additional limitation in our analysis is that we have assumed that the same risk estimates apply across all neighborhoods. For the long-term mortality effects examined, the Krewski et al. (2009) study used the citywide average PM2.5 concentrations across cities as the exposure contrast (i.e., the subjects in the same city are assigned the same PM2.5 level), and thus no within-city exposure variations were considered. Although Krewski et al. (2009) did examine modification by level of education, finding that mortality risk estimates increased with decreasing level of education, the published data do not include sufficient information to derive New York City neighborhood-specific concentration response functions.
Similarly, for the short-term effects studies, sub-urban C–R functions are generally not available for use in health impact assessments. National, multicity studies can examine effect modification by city- or county-level characteristics and provide evidence that socioeconomic status may modify short-term risks of ozone-attributable mortality (Bell and Dominici 2008) but do not quantify how neighborhood level concentration response functions vary across New York City neighborhoods. Within many cities, population sizes at the neighborhood level limit power for time-series or case-crossover analysis, resulting in larger uncertainty in risk estimates. Ongoing work is currently exploring the use of spatially stratified time-series models and other approaches for developing neighborhood level CR function estimates (Ito et al. 2009) that better reflect neighborhood differences in susceptibility to air pollution effects.
Without neighborhood level data on air pollution exposures that corresponded to neighborhood health incidence data, we elected to characterize subcounty exposures to ozone and PM2.5 as the inverse distance weighted average of nearby regulatory monitors (EPA VNA methodology). We recognize that regulatory monitoring networks are subject to spatial limitations that may not adequately characterize fine-scale concentration gradients found in urban areas. Ongoing and future work may help reduce spatial uncertainties in exposure, including applying data from high density monitoring networks with land-use regression (LUR) modeling (NYCDOHMH 2011b) or atmospheric modeling results at fine spatial scales (Wesson et al. 2011) that can supplement monitoring data. These methods are subject to their own limitations stemming from emissions inventories, source surrogate data, and fine-scale meteorology data. While beyond the scope of this paper, we have found that LUR-based neighborhood level exposure estimates for PM2.5 mass are more variable than the estimates based on inverse distance weighting (IDW) of regulatory monitors used in this paper (coefficient of variation = 0.13 and 0.07 for LUR-based and IDW estimates, respectively; NYC 2012). Future investigations will apply these estimates as multiple years of data become available and models are developed for other pollutants.
Conducting an air quality health burden analysis such as presented here includes the assumption that the same relationship between pollutant concentrations and health risk exists at levels well below the lowest measured levels in the epidemiological literature. While this introduces additional uncertainty in the shape of the dose–response curve at lower levels, available data does not suggest a health effect threshold in the range of concentrations relevant to our analysis. While this paper examined how overall health burden of PM2.5 and ozone varied with choices of spatial and temporal resolution, we recognize that our overall estimates of pollution-attributable impacts are sensitive to other method choices, including uncertainties explored elsewhere (Hubbell et al. 2009; Fuentes 2009).
Conclusions
We evaluated the sensitivity of estimates of citywide PM2.5 and ozone attributable health burden in New York City to spatial and temporal scale of baseline air pollution and health incidence data. While aggregated citywide estimates varied little by spatial resolution of air quality and baseline health incidence data, the finely stratified neighborhood-level analysis revealed significant sub-county variability in pollutant-attributable burdens, with significant disparities observed between low- and high-poverty neighborhoods. Comparisons of citywide impact estimates calculated from seasonal vs. annual incidence rates found the largest differences in ozone-attributable hospitalizations and emergency department visits due to simultaneous occurrence of lower-than-average baseline rates and high ozone concentrations, with the majority of ozone-attributable asthma emergency department visits occurring in the first half of the ozone season.
These findings indicate that the choice in methodology should ultimately be guided by the goal of the specific impact analysis. In analyses of broad control strategies that evenly affect urban areas spatially and temporally, county-level analysis may suffice, particularly if the planner is interested in evaluating citywide impacts of less variable health events, as detailed in the analysis of PM2.5-attributable mortality in New York City. However, in analyzing local policies that unevenly affect concentrations across neighborhoods within an urban area, our analyses demonstrate the importance of fine spatial resolution baseline incidence data that properly characterize subcounty differences in susceptibility and disparities by socio-economic status. Similarly, for health endpoints that vary temporally, such as in the example of ozone-attributable emergency department visits, using annualized incidence rates can bias citywide health impact estimates. These local scale assessments can help decision-makers target interventions in neighborhoods with relatively higher burdens of deaths and disease attributable to air pollution while providing useful data for community stakeholders in neighborhoods that suffer from relatively higher rates of morbidity than the county or city as a whole. Similarly, applying this level of analysis to future air quality policy development will help prioritize strategies that result in greater health benefits overall and reduce disparities in impacts across subpopulations.
References
Anderson BG, Bell ML (2010) Does one size fit all? The suitability of standard ozone exposure metric conversion ratios and implications for epidemiology. J Expo Sci Environ Epidemiol 20:2–12
Bell ML, Dominici F (2008) Effect modification by community characteristics on the short-term effects of ozone exposure and mortality in 98 US communities. Am J Epidemiol 167(8):986–997
Burtraw D, Palmer K, Bharvirkar R, Paul A (1998) Cost-effective reduction of NOx emissions from electricity generation. J Air Waste Manag Assoc 51:1476–1489
Cohen AJ, Anderson HR, Ostra B, Dev Pandey K, Krzyzanowski M, Kunzli N, Guschmidt K, Pope A, Romieu I, Samet JM, Smith K (2005) The global burden of disease due to outdoor air pollution. J Toxicol Environ Health A 68:1–7
Deguen S, Zmirou-Navier D (2010) Social inequalities resulting from health risks related to ambient air quality—a European review. Eur J Public Health 20(1):27–35
Dominici F, Peng RD, Barr CD, Bell ML (2010) Protecting human health from air pollution: shifting from a single-pollutant to a multipollutant approach. Epidemiol 21:187–194
US Environmental Protection Agency (1998) Regulatory impact analysis for the NOx SIP Call, FIP, and section 126, volume 2: Health and Welfare Benefits. EPA-452/R-98-003B. EPA Office of Air and Radiation
US Environmental Protection Agency (2006a) Air quality criteria for ozone and related photochemical oxidants. EPA/600/R-05/004aF. EPA Office of Research and Development
US Environmental Protection Agency (2006b) National ambient air quality standards for particle pollution. Available at: http://www.epa.gov/ttnecas1/ria.html. Accessed on: 23 Dec 2011
US Environmental Protection Agency (2008) Final ozone NAAQS regulatory impact analysis. EPA-452/R-08-003. EPA Office of Air Quality Planning and Standards
US Environmental Protection Agency (2009) Integrated science assessment for particulate matter EPA/600/R-08/139F. EPA Office of Research and Development
US Environmental Protection Agency (2010a) Quantitative health risk assessment for particulate matter. EPA-452/R-10-005. EPA Office of Air Quality Planning and Standards
US Environmental Protection Agency (2010b) Environmental Benefits Mapping and Analysis Program (BenMAP). Available at: http://www.epa.gov/air/benmap/ Accessed on: 23 Dec 2011
US Environmental Protection Agency (2011) Regulatory impact analysis for the federal implementation plans to reduce interstate transport of fine particulate matter and ozone in 27 states; correction of SIP approvals for 22 states. EPA-HQ-OAR-2009-0491. EPA Office of Air and Radiation
Fann N, Risley D (2011) The public health context for PM2.5 and ozone air quality trends. Air Qual Atmos Health. doi:10.1007/s11869-010-0125-0
Fann N, Lamson AD, Anenberg SC, Wesson K, Risley D, Hubbell BJ (2011a) Estimating the national public health burden associated with exposure to ambient PM2.5 and ozone. Risk Anal. doi:10.1111/j.1539-6924.2011.01630.x
Fann N, Roman HA, Fulcher CH, Gentile MA, Hubbell BJ, Wesson K, Levy JI (2011b) Maximizing health benefits and minimizing inequality: incorporating local-scale data in the design and evaluation of air quality policies. Risk Anal 31(6):908–922
Fiore A, Jacob DJ, Liu H, Yantosca RM, Fairlie TD, Li Q (2004) Variability in surface ozone background over the United States: implications for air quality policy. J Geophys Res. doi:10.1029/2003JD003855
Fuentes M (2009) Statistical issues in health impact assessments at the state and local levels. Air Qual Atmos Health 2(1):47–55
Huang Y, Dominici F, Bell ML (2005) Bayesian hierarchical distributed Lag models for summer ozone exposure and cardio-respiratory mortality. Environmetrics 16:547–562
Hubbell BJ, Hallberg A, McCubbin DR, Post E (2005) Health-related benefits of attaining the 8-Hr ozone standard. Environ Health Persp 113(1):73–82
Hubbell BJ, Fann N, Levy JI (2009) Methodological considerations in developing local-scale health impact assessments: balancing national, regional, and local data. Air Qual Atmos Health 2:99–110
Ito K, Thurston GD, Silverman RA (2007) Characterization of PM2.5, gaseous pollutants, and meteorological interactions in the context of time-series health effects models. J Expo Sci Environ Epidemiol 17:S45–S60
Ito K, Ross Z, Metzger K, Thurston GD, Matte T (2009) The temporal association between air pollution and asthma syndromic illness counts in New York City is modified by neighborhood traffic density. Presentation made at the ISEE meeting, Dublin, Ireland, August 25–29, 2009
Ito K, Mathes R, Ross Z, Nádas A, Thurston G, Matte T (2010) Fine particulate matter constituents associated with cardiovascular hospitalizations and mortality in New York City. Environ Health Persp. doi:10.1289/ehp.1002667
Krewski D, Jerrett M, Burnett RT, Ma R, Hughes E, Shi Y, Turner MC, Pope CA III, Thurston G, Calle EE, Thun MJ (2009) Extended follow-up and spatial analysis of the American Cancer Society Study Linking Particulate Air Pollution and Mortality. HEI Research Report 140. Health Effects Institute, Boston, MA
Laden F, Schwartz J, Speizer FE, Dockery DW (2006) Reduction in fine particulate air pollution and mortality: extended follow-up of the Harvard Six Cities study. Am J Resp Crit Care 173:667–672
Levy JI, Greco SL, Spengler JD (2002) The importance of population susceptibility for air pollution risk assessment: a case study of power plants near Washington, DC. Environ Health Persp 110(12):1253–1260
Matte TD, Cohen A, Dimmick F, Samet J, Sarnat J, Yip F, Jones N (2009) Summary of the workshop on methodologies for environmental and public health tracking of air pollution effects. Air Qual Atmos Health 2:177–184
Miranda ML, Edwards SE, Keating MH, Paul CJ (2011) Making the environmental justice grade: The relative burden of air pollution exposure in the United States. Int J Environ Res Public Health 8:1755–1771
Moolgavkar SH (2000) Air pollution and hospital admissions for chronic obstructive pulmonary disease in three metropolitan areas in the United States. Inhal Toxicol 12(Supp 4):75–90
National Research Council (2004) Air quality management in the United States. National Research Council of the National Academies, The National Academies Press, Washington DC
NESCAUM (2008) Public health benefits of reducing ground-level ozone and fine particle matter in the Northeast US: A Benefits Mapping and Analysis Program (BenMAP) study. Available at: http://www.nescaum.org/documents/benmap_report_1-16-08.pdf/. Accessed on: 5 April 2011.
New York City (2011) PLANYC 2030, update 2011. Available at: http://www.nyc.gov/planyc. Accessed on 12 Oct 2011
New York City (2012) Environmental public health and sustainability tracking portal. Available at: http://a816-dohbesp.nyc.gov/IndicatorPublic/EnterPortal.aspx. Accessed on 14 Aug 2012
New York City Department of Health and Mental Hygiene (NYCDOHMH) (2011) Air pollution and the heath of New Yorkers: the impact of fine particles and ozone. Available at: http://www.nyc.gov/html/doh/downloads/pdf/eode/eode-air-quality-impact.pdf. Accessed: 3 Aug 2011
New York City Department of Health and Mental Hygiene (NYCDOHMH) (2011b) The New York City community air survey: results from year one monitoring, 2008–2009. Available at: http://www.nyc.gov/html/doh/downloads/pdf/eode/comm-air-survey-report.pdf. Accessed: 14 Aug 2012
New York State Department of Environmental Conservation, Division of Air Resources (NYSDEC) (2011) Air quality management plan, June 2010 Draft. Available at: http://www.dec.ny.gov/docs/air_pdf/aqmpfinal610.pdf. Accessed on 3 Aug 2011.
NYCDOHMH DES. NYCDOHMH Department of Epidemiological Services. Neighborhood population estimates, modified from US Census Bureau vintage population estimates, New York, NY. Metadata available at: http://gisbesp100/metadatalite/PrintLiteSummary.aspx?metadata_id=110. Accessed on 6 Oct 2011
Silverman RA, Ito K (2010) Age-related associations of fine particles and ozone with sever acute asthma in New York City. J Allergy Clin Immun 125(2):367–373
Silverman RA, Ito K, Stevenson L, Hastings HM (2005) The relationship of fall school opening and emergency department asthma visits in a large metropolitan area. Arch Pediatr Adolesc Med 159:818–823
Wesson K, Fann N, Morris M, Fox T, Hubbell B (2010) A multi-pollutant, risk-based approach to air quality management: case study for Detroit. Atmos Pollut Res 1:296–304
Zanobetti A, Franklin M, Koutrakis P, Schwartz J (2009) Fine particulate air pollution and its components in association with cause-specific emergency admissions. Environ Health 8:58. doi:10.1186/1476-069X-8-58
Acknowledgments
This study was supported by a grant to the New York City Department of Health and Mental Hygiene from the National Center for Environmental Health, Centers for Disease Control and Prevention. We gratefully acknowledge the assistance of Dr. Kazuhiko Ito of the New York City Department of Health and Mental Hygiene in his review of this paper.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kheirbek, I., Wheeler, K., Walters, S. et al. PM2.5 and ozone health impacts and disparities in New York City: sensitivity to spatial and temporal resolution. Air Qual Atmos Health 6, 473–486 (2013). https://doi.org/10.1007/s11869-012-0185-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-012-0185-4