Abstract
Summary
Adherence is now one of the major issues in the management of osteoporosis and several papers have suggested that vertebral fractures might be increased in patients who do not follow appropriately their prescriptions. This paper relates the strong relationship existing between adherence to anti-osteoporosis treatment and the risk of subsequent hip fracture.
Introduction
A study was performed to investigate adherence to bisphosphonate (BP) therapy and the impact of adherence on the risk of hip fracture (Fx).
Methods
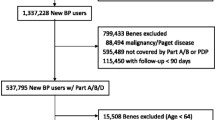
An exhaustive search of the Belgian national social security database was conducted. Patients enrolled in the study were postmenopausal women, naïve to BP, who received a first prescription of alendronate. Compliance at 12 months was quantified using the medication possession ratio (MPR). Persistence was calculated as the number of days from the initial prescription to a gap of more than 5 weeks after completion of the previous refill. A logistic regression model was used to estimate the impact of compliance on the risk of hip fracture. The impact of persistence on hip fracture risk was analysed using the Cox proportional hazards model.
Results
The mean MPR at 12 months was significantly higher among patients receiving weekly (n = 15.021) compared to daily alendronate (n = 14,136) (daily = 58.6%; weekly = 70.5%; p < 0.001). At 12 months, the rate of persistence was 39.45%. For each decrease of the MPR by 1%, the risk of hip Fx increased by 0.4% (OR: 0.996; CI95%:0.994–0.998; p < 0.001). The relative risk reduction for hip Fx was 60% (HR: 0.404;CI95%:0.357–0.457; p < 0.0001) for persistent compared to non-persistent patients.
Conclusion
These results confirm that adherence to current therapeutic regimens remains suboptimal.




Similar content being viewed by others
References
Hodgson SF, Watts NB, Bilezikian JP et al (2004) American Association of Clinical Endocrinologists medical guidelines for clinical practice for the prevention and treatment of postmenopausal osteoporosis: 2001 edition, with selected updates for 2003. Endocr Pract 9:544–564
Black DM, Cummings SR, Karpf DB et al (1996) Randomized trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture intervention trial research group. Lancet 348:1535–1541
Harris ST, Watts NB, Genant HK et al (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. JAMA 282:1344–1352
Reginster JY, Minne HW, Sorensen OH et al (2000) Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 11:83–91
Garnero P, Shih WJ, Gineyts E et al (1994) Comparison of new biochemical markers of bone turnover in late postmenopausal osteoporotic women in response to alendronate treatment. J Clin Endocrinol Metab 79:1693–1700
Harris ST, Gertz BJ, Genant HK et al (1993) The effect of short term treatment with alendronate on vertebral density and biochemical markers of bone remodelling in early postmenopausal women. J Clin Endocrinol Metab 76:1399–1406
Raisz L, Smith JA, Trahiotis M et al (2000) Short-term risedronate treatment in postmenopausal women: effects on biochemical markers of bone turnover. Osteopros Int 11:615–620
Delmas PD, Rizzoli R, Cooper C et al (2005) Treatment of patients with postmenopausal osteoporosis is worthwhile. Osteoporos Int 16:1–5
Chesnut CH, Skag A, Christiansen C et al (2004) Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 19:1241–1249
McClung MR, Geusens P, Miller PD et al (2001) Effects of risedronate on the risk of hip fracture in elderly women. Hip intervention program study group. N Engl J Med 344:333–340
Pols HA, Felsenberg D, Hanley DA et al (1999) Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Fosamax international trial study group. Osteoporos Int 9:461–468
Eastell R, Garnero P, Vrijens B et al (2003) Influence of patient compliance with risedronate therapy on bone turnover marker and bone mineral density response: the IMPACT study. Calcif Tissue Int 72:P297
Finigan J, Bainbridge PR, Eastell R (2001) Adherence to osteoporosis therapies. Osteoporos Int 12:S48–S49 P110
Yood RA, Emani S, Reed JI et al (2003) Compliance with pharmacologic therapy for osteoporosis. Osteoporos Int 14:965–968
Sebaldt RJ, Shane LG, Pham B et al (2004) Longer term effectiveness outcomes of noncompliance and nonpersistence with daily regimen bisphosphonate therapy in patients with osteoporosis treated in tertiary specialist care. Osteoporos Int 15(Suppl 1):S107P391SA
Caro JJ, Ishak KJ, Huybrechts KF et al (2004) The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int 15:1003–1008
Claxton AJ, Cramer J, Pierce C (2001) A systematic review of the associations between dose regimens and medication compliance. Clin Ther 23:1296–1310
Recker RR, Gallagher R, MacCosbe PE (2005) Effect of dosing frequency on bisphosphonate medication adherence in a large longitudinal cohort of women. Mayo Clin Proc 80:856–861
Cramer JA, Amonkar MM, Hebborn A et al (2005) Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin 21:1453–1460
Simon JA, Lewiecki EM, Smith ME et al (2002) Patient preference for once-weekly alendronate 70 mg versus once-daily alendronate 10 mg: a multicenter, randomized, open-label, crossover study. Clin Ther 24:1871–1886
Kendler D, Kung AWC, Fuleihan GE et al (2004) Patients with osteoporosis prefer once weekly to once daily dosing with alendronate. Maturitas 48:243–251
Cooper A, Drake J, Brankin E (2006) Treatment persistence with once-monthly ibandronate and patient support vs once-weekly alendronate: results from the PERSIST study. Int J Clin Pract 60:896–905
Siris ES, Harris ST, Rosen CJ et al (2006) Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc 81:1013–1022
Lo JC, Pressman AR, Omar MA et al (2006) Persistence with weekly alendronate therapy among postmenopausal women. Osteoporos Int 17:922–928
Motheral B, Fairman KA (1997) The use of claims databases for outcomes research: rationale, challenges, and strategies. Clin Ther 19:346–366
Ray WA, Griffin MR, Fought RL et al (1992) Identification of fractures from computerized Medicare files. J Clin Epidemiol 45:703–714
Clowes JA, Peel NF, Eastell R (2004) The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab 89:1117–1123
McCombs JS, Thibaud P, McLaughlin-Miley C et al (2004) Compliance with drug therapies for the treatment and prevention of osteoporosis. Maturitas 48:271–287
Huybrechts KF, Ishak KJ, Caro JJ (2006) Assessment of compliance with osteoporosis treatment and its consequences in a managed care population. Bone 38:922–928
Farmer KC (1999) Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther 21:1074–1090
Steiner JF, Prochazka AV (1997) The assessment of refill compliance using pharmacy records: methods, validity, and application. J Clin Epidemiol 50:105–116
Sikka R, Xia F, Aubert RE (2005) Estimating medication persistency using administrative claims data. Am J Manag Care 11:449–457
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rabenda, V., Mertens, R., Fabri, V. et al. Adherence to bisphosphonates therapy and hip fracture risk in osteoporotic women. Osteoporos Int 19, 811–818 (2008). https://doi.org/10.1007/s00198-007-0506-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-007-0506-x