Article Text
Abstract
Background We analysed the impact of breastfeeding, antiretroviral drugs and health service factors on cumulative (6 weeks to 18 months) vertical transmission of HIV (MTCT) and ‘MTCT-or-death’, in South Africa, and compared estimates with global impact criteria to validate MTCT elimination: (1) <5% final MTCT and (2) case rate ≤50 (new paediatric HIV infections/100 000 live births).
Methods 9120 infants aged 6 weeks were enrolled in a nationally representative survey. Of 2811 HIV-exposed uninfected infants (HEU), 2644 enrolled into follow-up (at 3, 6, 9, 12, 15 and 18 months). Using Kaplan-Meier analysis and weighted survey domain-based Cox proportional hazards models, we estimated cumulative risk of MTCT and ‘MTCT or death’ and risk factors for time-to-event outcomes, adjusting for study design and loss-to-follow-up.
Results Cumulative (final) MTCT was 4.3% (95% CI 3.7% to 5.0%); case rate was 1290. Postnatal MTCT (>6 weeks to 18 months) was 1.7% (95% CI 1.2% to 2.4%). Cumulative ‘MTCT-or-death’ was 6.3% (95% CI 5.5% to 7.3%); 81% and 62% of cumulative MTCT and ‘MTCT-or-death’, respectively, occurred by 6 months. Postnatal MTCT increased with unknown maternal CD4-cell-count (adjusted HR (aHR 2.66 (1.5–5.6)), undocumented maternal HIV status (aHR 2.21 (1.0–4.7)) and exclusive (aHR 2.3 (1.0–5.2)) or mixed (aHR 3.7 (1.2–11.4)) breastfeeding. Cumulative ‘MTCT-or death’ increased in households with ‘no refrigerator’ (aHR 1.7 (1.1–2.9)) and decreased if infants used nevirapine at 6 weeks (aHR 0.4 (0.2–0.9)).
Conclusions While the <5% final MTCT target was met, the case rate was 25-times above target. Systems are needed in the first 6 months post-delivery to optimise HEU health and fast-track ART initiation in newly diagnosed mothers.
- Public health
- child health
- epidemiology
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
INTRODUCTION
Since 2002, the coverage and intensity of national programmes to prevent vertical HIV transmission (prevention of mother-to-child transmission—PMTCT) have increased in high HIV prevalence, low- and middle-income countries (LMICs). Early and long-term triple antiretroviral therapy (ART) among women living with HIV (WLHIV) in research sites reduces vertical transmission of HIV (MTCT), improving infant HIV-free survival up to 48 weeks.1–4 Consequently, in countries where 90% of the world’s population of pregnant WLHIV reside (global plan priority countries), lifelong maternal ART, known as PMTCT Option B+, has been introduced to reduce MTCT.5 Data from national programmes have been skewed towards measuring early (birth or 6 weeks postpartum) PMTCT impact as early populations are easier to access.6 7 Consequently, we know that intrauterine MTCT (measured at birth) and 6-week MTCT can be reduced to 0.9% and 1.1%, respectively.8 However, in LMICs where breastfeeding is normative, MTCT at the end of breastfeeding is mostly modelled.9 A reliable measure of final MTCT (at the end of breastfeeding) is imperative to validate the elimination of MTCT (EMTCT) as a public health problem.10 Two EMTCT impact criteria have been identified for validation: (1) <5% final MTCT in breastfeeding populations and (2) ≤50 new paediatric infections per 100 000 live births by the end of breastfeeding (case rate). Both need to be sustained for 1 year.10 Consequently, measuring long-term outcomes of HIV-exposed infants (HEI) is critical. In this paper, we report the impact of breastfeeding, maternal ART and health service factors on postnatal MTCT (>6 weeks to 18 months), cumulative (6 weeks to 18 months) MTCT and ‘MTCT-or-death”.
METHODS
Study design and sample size
We conducted a prospective observational cohort study. HEI were identified through infant HIV-antibody testing (maternal HIV-antibody crosses the placenta and is detectable in infants’ blood). Enrolment occurred at 6 weeks (range 4–8 weeks) postnatal age (baseline visit), as part of a nationally representative, cross-sectional, early PMTCT effectiveness survey (29 October 2012–31 May 2013)7 11: 2314 and 1097 HEI were required to estimate 18-month cumulative MTCT and ‘MTCT-or-death’, respectively, with a relative precision of 30%, design effect of 2 and assuming 30% loss to follow-up at 18 months.
Data collection methods
Nurse data collectors (DCs), trained on standardised procedures over 5 days, collected data (October 2012–September 2014). Mothers or caregivers and their infants were sampled systematically (in facilities with long immunisation queues) or consecutively (in small facilities). Infants needing emergency care were excluded. HIV-antibody–positive infants or infant born to mothers who reported being HIV positive were tested for HIV infection. The latter were recruited into follow-up at baseline. Self-reported HIV-negative mothers whose infants tested HIV-antibody–positive were also eligible for follow-up. DCs arranged follow-up appointments coinciding with the child’s routine follow-up visits, at 3, 6, 9, 12, 15 and 18 months, or until the child died or for one visit after an infant’s HIV-positive diagnosis, whichever came first. At each follow-up, DCs conducted structured face-to-face interviews, corroborated reported information with patient-held routine infant Road-to-Health Cards/Booklets (RTHB) and pictorial food-medication-hospitalisation study diaries, and drew infant blood for HIV testing. Infant feeding questions evaluated the intake of individual items (yes/no response to each) over the previous 24 hours and 7 days prior to these 24 hours (8-day recall in total). Data were captured on mobile telephones and uploaded real-time onto a password-protected web-based interface. Trained supervisors monitored fieldwork.
PMTCT national guideline context
From 1 April 2010 to 31 March 2013, South Africa implemented WHO PMTCT ‘Option A’ policy (pregnant WLHIV received ART if CD4 cell count ≤350 cells/mm3 or antiretroviral prophylaxis (ARVs) from 14 weeks’ gestation; HEI received nevirapine (NVP) prophylaxis for 6 weeks if not breastfeeding or until 1 week post-breastfeeding cessation.)12 From 1 April 2013, South African adopted WHO PMTCT ‘Option B’ policy (pregnant or lactating WLHIV received ART lifelong if CD4 cell count ≤350 cells/mm3 or until 1 week post-breastfeeding cessation; HEI received NVP prophylaxis for 6 weeks).13 National guidelines recommended routine HIV virologic testing among HEI at 6 weeks post-delivery and 6 weeks post-breastfeeding cessation, and HIV-antibody testing at 18 months post-delivery.
Study HIV testing procedures
At baseline, HEI were identified using HIV-antibody testing on infant-dried blood spots (iDBS), at the National Institute for Communicable Diseases (NICD), Johannesburg.7 11 Antibody-positive specimens or specimens from infants whose mothers reported being HIV positive were tested for HIV infection using qualitative total nucleic acid (TNA) PCR (COBAS AmpliPrep/COBAS TaqMan (CAP/CTM) Qualitative assay version 1.0, Roche Diagnostics, Branchburg, NJ). At follow-up (3, 6, 9, 12 and 15 months), iDBS from HIV-exposed uninfected (HEU) infants were tested using TNA HIV (PCR) (COBAS AmpliPrep/COBAS TaqMan (CAP/CTM) Qualitative assay version 1.0, Roche Diagnostics). At 18 months, one (if negative) or two (if positive) serial rapid HIV tests performed by routine (not study) nurses, as per routine protocol, identified HIV infection. Until 31 March 2014, SD Bioline HIV 1/2 3.0 Test D3FK16 was used as the first rapid test and First Response HIV 1–2.0 as the second test. From 1 April 2014, Advanced Quality (Armada Health (Pty)) and One Step Advanced Quality (Titma Health (Pty)) were used as the first rapid test and Abon (Fit Healthcare and Diagnostics (Pty)) as the second test. These tests all have sensitivities and specificities ≥99%.
Ethics
The protocol was approved by the institutional review board of the South African Medical Research Council (SAMRC) and reviewed by the Centers for Disease Control and Prevention (CDC), Atlanta. All mothers/caregivers signed informed consent. Laboratory results were returned to mothers through routine services, assisted by study staff as needed. During all study visits, mother and infants not in care were referred to routine services. No personal identifiers were included in study databases.
Data analysis
Data analysis was conducted using SAS version 9.4 2002–2012 by SAS Institute, Cary, NC, USA) and Stata 13. Infants identified as being HIV exposed and infected (HIV serology and PCR positive) at baseline were included in the calculation of cumulative (6 weeks to 18 months) MTCT and ‘MTCT-or-death’ All analyses were weighted for population live births, non-consent (mothers who did not consent to follow-up) and loss to follow-up.
Risk of cumulative (6 weeks to 18 -months) MTCT
A competing risk model was performed to measure cumulative MTCT (6 weeks to 18 months), with death as the competing risk. At each time point, we calculated MTCT as the weighted proportion of HEU infants whose iDBS was TNA PCR positive. Additionally, we calculated interval-specific MTCT incidence for ART use reported at 6 weeks and breastfeeding as a time-dependent variable, using 8-day recall of infant feeding practices from the day of interview.
Risk of cumulative (6 weeks to 18 months) ‘MTCT-or-death’
The time to first occurrence of either MTCT (HIV-positivity) or death was used to estimate the cumulative incidence of ‘MTCT-or-death’ (6 weeks to 18 months). A weighted Kaplan-Meier analysis was conducted, and the 95% CIs were adjusted for survey design effect. The design effect was that of a domain-based survey logistic regression model of ‘MTCT or death’ within the cohort up to 18 months.
Risk factors for postnatal (after 6 weeks to 18 months) MTCT and cumulative (6 weeks to 18 months) ‘MTCT-or-death’
To evaluate risk factors for postnatal MTCT, we excluded infant HIV infections measured at baseline (6 weeks). A survey-domain-based Cox proportional hazards model with time-dependent and time-independent covariates was used with finite sampling correction factor for the primary sampling unit (facilities). We tested the widely known association between feeding practices and infant outcome, and the potential association between maternal ART use and infant outcome with postnatal (after 6 weeks to 18 months) MTCT as the main outcome. This analysis adjusted for several factors: (1) maternal and infant characteristics (maternal age, infant gender and infant NVP receipt), (2) health service factors (hospital birth, mother tested for CD4 count and documentation of maternal HIV on the RTHB), and (3) socio-economic status (access to piped water, toilet, refrigerator, transport, food and maternal single status). Using 8-day recall, we defined any breastmilk exposure as any infant ingestion of breastmilk at that specific time point; exclusive breastfeeding (EBF) as breastmilk only with the exception of prescribed medicines or oral rehydration solution; mixed breastfeeding (MBF) as breastmilk with nutritive or non-nutritive liquids or solids and no breastfeeding (NBF) as no reported breastmilk ingestion. Maternal ART exposure was defined as maternal self-reported ART use at each follow-up point. The model for postnatal MTCT (N=34 events) included maternal ART as a time-dependent factor and EBF, MF or NBF, documented at baseline (6 weeks), to capture the risk associated with early feeding practice, given that most transmission occurred before 6 months post-delivery.
The final model for cumulative (6 weeks to 18 months) ‘MTCT-or-death’ (the inverse of HIV-free survival) included ART and any breastmilk exposure (EBF, MBF, NBF) as time-dependent variables, on our assumption that ART-use and feeding practice throughout this period affects outcome. Cross-tabulations of the time-dependent variables are presented to describe the transitions in feeding practices and ART over time.
ROLE OF THE FUNDING SOURCE
The South African National AIDS Council and Global Fund funded the study without undue influence. CDC, UNICEF, the NICD, and National Department of Health (NDOH) provided funding and technical support for protocol development and data analysis, mobile data collection, laboratory procedures and study contextualisation, respectively, without undue influence. The SAMRC provided infrastructural support and partial support for A Goga’s time.
RESULTS
Of 2878 HEI, 67 were HIV positive at 6 weeks post-delivery; thus, 2811 HEU infants and their caregivers were eligible for follow-up after 6 weeks. Of these, 167 (5.9%) caregivers refused to participate in the follow-up visits (figure 1). Thus, 2644 infants were enrolled for 18-month follow-up; 84.6% (2238) of enrolled infants had at least one face-to-face postnatal follow-up, and 96% (2538) were tracked for survival. The proportion of eligible infants with interview and iDBS data at 3, 6, 9, 12, 15 and 18 months was 68%, 65%, 64% 68%, 66% and 71% (n=1797 at 18 months), respectively.
Profile 2012–2014 study population.
Eligible mothers who did not consent to infant follow-up were less likely to receive maternal antiretroviral drugs (ARVs), p<0.001, and were significantly younger (p=0.03) compared with mothers who consented to follow-up. Mothers who consented but did not return for infant follow-up were less likely to receive maternal ARVs (p<0.001) and less likely to come from Northern or Western Cape provinces. There were no significant differences in feeding patterns at 6 weeks post-delivery between infants included in this analysis and those excluded (no consent for follow-up or lost to follow-up).
Infant feeding practices, maternal ART- and infant NVP-use between 6 weeks and 18 months
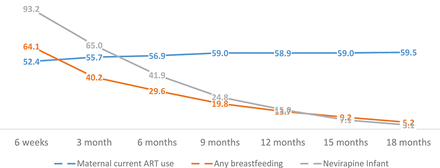
The weighted prevalence of any breastfeeding decreased from 64.1% at 6 weeks, to 5.2% at 18months (figure 2 and online supplementary table 1). The weighted prevalence of maternal ART-use was 52.4% at 6 weeks and 59.5% at 18 months (p>0.05 (figure 2 and online supplementary table 1). Infant NVP-use among all infants was 93.2% (95% CI 91.8% to 94.7%) at 6 weeks decreasing to 3.1% (95% CI 2.3% to 3.9%), at 18 months (figure 2 and online supplementary table 1). Among breastfeeding infants, NVP use was 93.8% at 6 weeks, 85.4% at 3 months and 22.4% at 18 months (data not shown). Among breastfeeding infants whose mothers were not on ART, a higher percentage (96.7%, 95% CI 94.7% to 98.6%) were on NVP at 3 months, decreasing to just below 70% by 12 months (data not shown).
Supplemental material
Cumulative (6 weeks to 18 months) and postnatal (after 6 weeks to 18 months) MTCT
Cumulatively (6 weeks to 18 months), 101 HIV infections were detected among HEI. Of these, 67 infant HIV infections were detected at 6 weeks post-delivery and 34 were detected during postnatal follow-up until 18 months. Using a competing risk approach with death as the competing risk, the cumulative MTCT risk (including the 6-week result) was 4.3% (CI 3.7% to 5.0%) (figure 3 and online supplementary table 2). Assuming an antenatal HIV prevalence of 30%, this translates into a case rate of 1290 new paediatric infections per 100 000 live births (30% maternal HIV prevalence×100 000 live births×4.3% MTCT at final endpoint). Among infants who were HIV infected by 18 months (n=101), 60% of the cumulative MTCT risk occurred by 6 weeks (range 4–8 weeks) post-delivery; 81% occurred in the first 6 months post-delivery. The postnatal (after 6 weeks to 18 months) MTCT risk was 1.7% (95% CI 1.2% to 2.4%). All infants with postnatal HIV acquisition had breastmilk exposure before or at the time of their HIV diagnosis. Twenty-two postnatally infected infants had data on complete breastfeeding cessation. Of these, six reported complete breastfeeding cessation to the yes/no question, but all reported mixed feeding when specifically asked about previous 8-day feeding practices at the visit prior to the infant’s HIV diagnosis. Although 10 of the 22 HEI reported not breastfeeding over the previous 8 days at the 6-week visit, 5 were mixed feeding at 3 months and tested HIV positive at 3 months. Only 1 of these 10 mothers was on ART. Two HEI were also reportedly not breastfeeding at the time of infant HIV diagnosis at 12 months (both last reported breastfeeding at 6 months), but both reported breastfeeding at 15 months. One of the 22 breastfed at 6 weeks, 3, 9 and 15 months, with no reported breastfeeding at 6 and 12 months, had equivocal HIV test results at 6 weeks, 3, 6, and 12 months and tested HIV positive at 15 months.
Estimation of interval (weeks)-specific MTCT percentage by corresponding reported breastfeeding practice and start of ART initiation reported at 6 weeks post-delivery
Survey Cox regression model of factors associated with postnatal (6 weeks to 18 months) MTCT and ‘MTCT-or-death’
Weighted percentage of maternal ART-use, infant feeding practices and infant nevirapine use among mothers with consecutive follow-up visits between 6 weeks and 18 months. ART, antiretroviral therapy.
Postnatal MTCT cumulative incidence curve (%) with 95% CI. MTCT, mother-to-child transmission of HIV.
Cumulative incidence curve (%) of MTCT-or-death including the 6-week time point with 95% CI. MTCT, mother-to-child transmission of HIV.
Cumulative Incidence curve of postnatal MTCT-or-death (excluding the 6-week time point) with 95% confidence intervals
Supplemental material
MTCT by feeding practice and ART group
Unadjusted interval-specific analysis relating MTCT to the corresponding reported feeding practice demonstrated that MTCT at baseline (6 weeks) was 4.7% (95% CI 2.8% to 8.9%) among mothers who reported MBF, 2.9% (95% CI 2.0% to 4.2%) among mothers reporting EBF and 1.3% (95% CI 0.8% to 2.4%) among mothers reporting NBF (table 1). Using 8-day recall data, we documented postnatal MTCT among mothers who reported not breastfeeding (table 1). The unadjusted risk of MTCT by ART initiation period reported at 6 weeks was lowest among women who initiated ART preconception (table 1). Numbers in these breastfeeding and ART groups were too small for additional modelling. Breastfeeding prevalence decreased approximately fivefold, from 29.6% at 6 months to 5.2% at 18 months; cumulative MTCT increased by approximately 0.2% every 3 months, and approximately 1.1-fold from 6 to 18 months (3.9% at 6 months to 4.3% at 18 months).
‘MTCT or death’
For the composite outcome ‘MTCT-or-death’ (whichever came first), 62 of 2644 HEI died and 34 acquired HIV between 6 weeks and 18 months. Thirty of the 62 infants died before we determined their final HIV-status but were PCR negative at the last visit. The weighted cumulative risk of ‘MTCT-or-death’ (6 weeks to 18 months) was 6.3% (95% CI 5.5% to 7.3%) (figure 4A and online supplementary table 3). Sixty-two per cent of cumulative ‘MTCT-or-death’ had occurred by 6 months post-delivery. After the 6-week time point, ‘MTCT-or-death’ occurred in 3.7% of infants (figure 4B and online supplementary table 3).
Supplemental material
Risk factors for postnatal (6 weeks to 18 months) MTCT and ‘MTCT-or-death’
Time-dependent maternal ART use (time-dependent indicator) was not significantly associated with postnatal MTCT or ‘MTCT-or-death’, adjusted HR (aHR)=0.6 (95% CI 0.3 to 1.2) and 0.8 (95% CI 0.6 to 1.2), respectively (table 2). The risk of postnatal MTCT significantly increased if the mother did not receive her CD4 cell count result (aHR=2.7 (95% CI 1.5 to 5.6)), if there was no documentation of infant HIV-exposure on the RTHB, aHR=2.2 (95% CI 1.0 to 4.7), or if the infant reported mixed feeding or EBF at 6 weeks using 8-day recall data (aHR=3.7 (95% CI 1.2 to 11.4), and 2.3 (95% CI 1.0 to 5.2), respectively) (table 2). However, mixed feeding and breastfeeding were not significant risk factors for ‘MTCT-or-death’, aHR 0.9 (95% CI 0.6 to 1.3) and aHR 1.6 (95% CI 0.8–2.9), respectively.
‘MTCT-or-death’ was significantly increased in households without a refrigerator’ (aHR=1.74 (95%CI 1.06 to 2.88)), and significantly decreased by infant NVP use at 6 weeks (aHR=0.4 (95% CI 0.18 to 0.88)) (table 2).
DISCUSSION
We demonstrate that cumulative (6 weeks to 18 months) MTCT and ‘MTCT-or-death’ was 4.3% (95% CI 3.7% to 5%) and 6.3% (95% CI 5.5% to 7.3%), respectively, in the South African national PMTCT programme by 2014. While this meets the <5% WHO final MTCT impact criterion, the 30% antenatal HIV prevalence14 translates into a case rate of 1290 new paediatric HIV infections per 100 000 live births—more than 25 times the target. In previous analyses, we demonstrated that maternal HIV prevalence drives the paediatric case rate.15 16
Eight-two per cent of cumulative MTCT and 62% of cumulative ‘MTCT-or-death’ occurred by 6 months post-delivery, and approximately 40% of cumulative MTCT was attributable to postnatal MTCT in the context of 64.1% breastfeeding prevalence at 6 weeks, decreasing to 29.6% at 6 months. Consequently, strengthening routine maternal and child primary healthcare, including breastfeeding support, viral suppression for WLHIV and early infant HIV testing for HEI will be pivotal to improving infant HIV-free survival henceforth.17
With 6.3% cumulative ‘MTCT or death’, 93.7% of HEI remaining in follow-up were alive and HIV-free by 18 months. Our study demonstrated a marked improvement in HIV-free survival compared with the 77.7% (95% CI 72.5% to 87.0%) HIV-free survival at 24 months measured in two South African sites in the era of single-dose NVP.18 Our findings were similar to the 91.9% (95% CI 90.3% to 93.3%) HIV-free survival measured at 9–14 months in a nationally representative community-based study conducted prior to PMTCT Option B+ implementation in Rwanda, a low HIV-prevalence setting.19 Our data thus demonstrate the impact of PMTCT Option A and B on 18-month MTCT and HIV-free survival in a high HIV-prevalence setting. These successes were achieved despite gaps in coverage of maternal ART (<60% at 18 months) and infant NVP prophylaxis among breastfed infants (73% at 6 months and 22.2% at 18 months), and in the context of declining breastfeeding prevalence. Our findings demonstrate lower early and final MTCT compared with Swaziland (2–3% at 6 weeks post-delivery, increasing to 12–15% at 18 months) and Zimbabwe 8.8% (95% CI 6.9% to 11.1%) at 9–18 months in 201120 21 and 7% at 18 months in 2013–2014.22 Transmission in Zimbabwe ranged from 3% with preonception maternal ART-initiation to 19.4% with no maternal or infant antiretroviral exposure. We did not measure maternal viral load or gather detailed information on maternal ART; thus, the relative contributions of breastfeeding, ART and maternal HIV are difficult to disentangle.23
Although infant NVP use among breastfeeding infants decreased to 22% by 18 months, extended NVP use among breastfeeding infants whose mothers were not on ART was 96.7%, at 3 months, decreasing to just below 70% by 12 months. Our low (5%) 18-month MTCT may be the result of low breastfeeding prevalence and high extended NVP coverage among breastfeeding infants whose mothers were not on ART in the first 6 months of life.2 4 24 25 Our finding of postnatal MTCT among mothers who reported not breastfeeding may represent missed intrauterine or intrapartum infections, given that HIV diagnostic tests have lower sensitivity with increasing infant exposure to maternal ART26 or dynamic feeding practices postnatally. This, together with declining infant NVP prophylaxis among breastfed infants, illustrates a potential role for complementary preventative interventions such as neutralising antibodies for breastfeeding infants, which need investigation.
EBF or MBF, in the context of maternal ART and infant NVP use, was not significantly associated with an increased risk of ‘MTCT-or-death’, reinforcing breastfeeding as a child survival strategy in the context of maternal ART-use and viral suppression, validating the 2016 WHO update on HIV and infant feeding.27 The latter emphasises the importance of retention in care for mothers and babies and monitoring virologic suppression.27 The lack of protective association between postnatal MTCT or cumulative 18-month ‘MTCT-or-death’ and ART-use (regardless of feeding practice) could relate to poor adherence and low coverage of ART postnatally.28
Lastly, no documentation of infant HIV exposure status on patient-held records, and ‘mother not tested for CD4 cell count’ significantly increased the risk of postnatal MTCT, reminding us that biomedical interventions need to be implemented alongside health system strengthening initiatives.29
Limitations and strengths
We may have underestimated MTCT and MTCT-or-death as recruitment began at first immunisation visit, excluding early infant deaths, and being a close cohort, we excluded mothers with incident HIV infections postnatally. Furthermore, we used the WHO standard for HIV diagnosis at 18 months (rapid HIV testing), which, under field conditions, has a lower sensitivity for detecting recent infection than PCR. We did not have access to maternal viral loads, to investigate MTCT by viral load. The small number of postnatal HIV infections (n=34) limited extensive modelling. Although we account for two types of lost to follow-up (no consent and consent but no show) by inverse proportional weighting, these missing information may have underestimated MTCT. Notwithstanding this, we assessed national PMTCT effectiveness in a high HIV prevalence setting, which few high HIV-prevalence countries have been able to measure. The cohort was drawn from a nationally representative sample with high rates of consent (94.1%), survival tracing (96%) and 71% follow-up to 18 months. The analyses are weighted for live births, non-consent and loss to follow-up, thus reducing bias and allowing extrapolation to national level. While there may be considerable attenuation of the study group with differential non-inclusion and considerable amount of censoring, limiting the denominator used in the later events, our findings are comparable with other studies.
CONCLUSIONS
While the WHO target of <5% final MTCT was met, the estimated case rate (1290) was 25 times above target. Systems are especially needed to optimise the health of HEUs from deprived settings (eg, no refrigerator) in the first 6 months post-delivery. Breastfeeding was not a significant risk factor for ‘MTCT-or-death’. Given that we described transmissions among children with no reported breastfeeding, ART initiation should be fast-tracked in mothers newly diagnosed with HIV infection postnatally even if they do not report breastfeeding.
What is already known on this subject
Most global plan priority countries do not routinely monitor long-term national PMTCT programme effectiveness; they rely on modelling.
In 2009, Ruton et al estimated HIV-free survival in Rwanda, at 9–24 months as 91.9% (95% CI 90.4% to 93.3%) following a nationally representative household survey (n=1448 HIV-exposed uninfected infants), conducted when single-dose nevirapine was policy.
In 2012, Buzdugan et al surveyed 1081 mother-HIV exposed infant pairs in Zimbabwe, from 157 randomly selected health facilities within 5 of the 10 provinces. 9- to 18-month MTCT ranged from 8.8% (95% CI 6.9% to 11.1) to 10.1% (95% CI 7.7% to 13.1%) and HIV-free survival ranged from 89.8% (95% CI 86.8 to 92.2) to 90.9% (95% CI 88.7% to 92.7%), depending on how PMTCT exposure was defined.
In 2013, Dinh et al measured national MTCT before scale up to PMTCT Option B+ (n=1172 mother–HIV-exposed infant pairs at 18 months). Participants were recruited from 151 immunisation clinics nationally. MTCT was 7% by 18 months, with 50% MTCT measured by 6 weeks, and an additional 25% by 6 months. Post-delivery MTCT was lowest (1.8%) among mothers who started ART preconception (1.2%) versus pregnancy (2.7%). HIV-free survival was not estimated in this analysis.
As far we know, no other studies have been undertaken to assess long-term national PMTCT effectiveness.
What this study adds
We demonstrate attainment of the first impact criteria for final MTCT elimination (MTCT <5%) in South Africa, by 2014. This was achieved notwithstanding 60% maternal ART coverage at 18 months, and 72% and 22% infant nevirapine propylaxis coverage among breastfeeding infants at 6 and 18-months, respectively.
The case rate was estimated as 1290 which is 25 times higher than the global target, demonstrating that South Africa is not on track to eliminate MTCT.
We also measured 93.7% HIV-free survival (95% CI 92.7% to 94.5%), and 1.7% postnatal MTCT after 6 weeks through 18 months.
Breastfeeding was not a risk factor for ‘MTCT-or-death’, illustrating the continued protective effect of breastfeeding under antiretroviral cover, in countries with high background mortality.
We also demonstrate the importance of regular follow-up and care during the first 6 months of life as 82% of cumulative (6 weeks to 18 months) MTCT and 62% of cumulative ‘MTCT-or-death’ occurred by 6 months post-delivery.
Policy implications
Our findings substantiate the 2016 WHO updated guidance to harmonise infant feeding recommendations between HIV-positive and HIV-negative women under ART cover and viral suppression.
Our findings illustrate the critical need to (i) strengthen the routine postnatal care platform in the first 6 months of life, including breastfeeding under ART cover and maternal viral suppression; (ii) implement regular infant HIV testing in the first 6 months of life, or at least at 6-months, rather than waiting until 18 months and (iii) know maternal blood results, and document maternal HIV status on patient-held health cards.
Inter-sectoral interventions are needed to improve socio-economic status, and special care and attention is needed for HIV-exposed uninfected infants from deprived backgrounds (no refridgerator) to optimise their HIV-free survival.
Acknowledgments
The authors thank the funders and collaborators listed below, and mothers and infants enrolled in the study.
REFERENCES
Footnotes
Contributors AEG—principal investigator (PI) on the study—conceptualised the paper, oversaw all fieldwork, interpreted data, provided the theoretical background to guide statistical analysis, drafted the paper, incorporated all co-author and CDC comments, finalised the paper and approved the final version. CJL—hief statistician—calculated sample size, conducted all analyses, wrote statistical analysis section, contributed to all versions of the paper and approved the final version. DJJ—PI on the study, was in charge of the mobile technology used to gather data, assisted with data interpretation, commented on all versions of the paper and approved the final version. VR assisted with data management and interpretation, commented on all versions of the paper and approved the final version. NN conducted data management and cleaning, assisted with data interpretation, commented on all versions of the paper and approved the final version. GGS assisted with training on dried blood spots drawing and with systems to return results, guided data analysis, and interpretation, commented on all versions of the paper and approved the final version. AP oversaw all laboratory work in accordance with standardised operating procedures, write the laboratory section of the paper, commented on all versions of the paper and approved the final version. WC assisted with data interpretation, commented on all versions of the paper and approved the final version of the paper. SB assisted with data interpretation, commented on all versions of the paper and approved the final version of the paper. NM—logistics manager on the study—assisted with data interpretation, commented on all versions of the paper and approved the final version of the paper. TR—provincial coordinator on the study—assisted with data interpretation, commented on all versions of the paper and approved the final version of the paper. VM—provincial coordinator on the study—assisted with data interpretation, commented on all versions of the paper and approved the final version of the paper. YS oversaw the development of the mobile technology systems for data collection and functioning thereof, assisted with data interpretation, commented on all versions of the paper and approved the final version of the paper. YP assisted with conceptualisation of the study and data interpretation, commented on all versions of the paper and approved the final version of the paper. T-HD—PI on the study and is senior author on the paper—assisted with setting up systems for cohort monitoring, assisted with data management and data interpretation, commented on all versions of the paper and approved the final version.
Funding This study was primarily funded by the US President’s Emergency Plan for AIDS Relief (PEPFAR) through the US Centers for Disease Control and Prevention (CDC) under terms of cooperative agreement number 1U2GPS001137 and 1U2GGH001150. UNICEF, the South African Medical Research Council, the South African National AIDS Council, the National Institute of Communicable Diseases, the National Department of Health and the Global Fund (through the National Department of Health) also provided funding support through collaboration agreements. The South African National Research Foundation supported D Jackson during the time of this study.
Disclaimer The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies.
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available upon reasonable request.