Article Text
Abstract
Background Effective condom use can prevent sexually transmitted infections (STIs) and unwanted pregnancy. We conducted a systematic review and methodological appraisal of randomised controlled trials (RCTs) of interventions to promote effective condom use.
Methods We searched for all RCTs of interventions to promote effective condom use using the Cochrane Infectious Diseases Group's trials register (Oct 2006), CENTRAL (Issue 4, 2006), MEDLINE (1966 to Oct 2006), EMBASE (1974 to Oct 2006), LILACS (1982 to Oct 2006), IBSS (1951 to Oct 2006) and Psychinfo (1996 to Oct 2006). We extracted data on allocation sequence, allocation concealment, blinding, loss to follow-up and measures of effect. Effect estimates were calculated.
Results We identified 139 trials. Seven out of ten trials reported reductions in ‘any STI’ with five statistically significant results. Three out of four trials reported reductions in pregnancy, although none was statistically significant. Only four trials met all the quality criteria. Trials reported a median of 11 (IQR 7–17) outcome measures. Few trials used the same outcome measure. Altogether, 10 trials (7%) used the outcome ‘any STI’, 4 (3%) self-reported pregnancy and 22 (16%) used ‘condom use at last sex’.
Conclusions The results are generally consistent with modest benefits but there is considerable potential for bias due to poor trial quality. Because of the low proportion of trials using the same outcome the potential for bias from selective reporting of outcomes is considerable. Despite the public health importance of increasing condom use there is little reliable evidence on the effectiveness of condom promotion interventions.
- Contraception RB
- contraception SA
- sexual behaviour
- sexual health
- sexually transdis
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-commercial License, which permits use, distribution, and reproduction in any medium, provided the original work is properly cited, the use is non commercial and is otherwise in compliance with the license. See: http://creativecommons.org/licenses/by-nc/2.0/ and http://creativecommons.org/licenses/by-nc/2.0/legalcode.
Statistics from Altmetric.com
Introduction
Unsafe sex is believed to be the second most important risk factor for disease, disability or death in the poorest countries of the world, and the ninth most important factor in developed countries.1 Effective condom use has the potential to prevent sexually transmitted infections (STIs), including HIV, and unwanted pregnancy.2 3 However, condom effectiveness is lower than condom efficacy due to non use, inconsistent use and the incorrect application of condoms.4 5 Therefore, interventions that promote effective condom use have considerable potential to improve public health.
Interventions to increase effective condom use have addressed condom design, access to condoms, condom use behaviours and condom-related legislation. Existing systematic reviews of the effectiveness of interventions to promote effective condom use have examined specific population groups or interventions,6–8 but to date there has been no comprehensive systematic review of randomised controlled trials (RCTs) of interventions to promote effective condom use.
Although systematic reviews of RCTs are considered to provide the most valid and reliable evidence of the effectiveness of healthcare interventions, recent studies have drawn attention to the effect of trial quality and selective publication on their results.9 Selective publication of trials has been recognised as a potent threat to validity for many years but more recently the importance of selective publication of trial outcomes has also been highlighted.10–13 We report a systematic review and methodological appraisal of RCTs of interventions to promote effective condom use.
Methods
Inclusion criteria
We included all RCTs of interventions to promote effective condom use regardless of publication status or language. Participants were men and women of any age. Interventions were any measure intended to increase effective condom use. Trials of female condoms or those comparing latex and non-latex condoms were excluded because they have been reviewed previously.14 Primary outcomes were the occurrence of pregnancy and STIs. Secondary outcomes were measures of condom use, including condom use at first sexual intercourse, condom use at last sexual intercourse, 100% condom use, frequency of condom use, frequency of unprotected sex, proportion of episodes of sex protected, condom use scales and refusal of sexual intercourse if condom not used. Secondary outcomes for condom failure outcomes included clinical breakage, non-clinical breakage and full or partial slippage rates.
Search strategy
We searched the Cochrane Infectious Diseases Group's trials register (Oct 2006), CENTRAL (Issue 4, 2006), MEDLINE (1966 to Oct 2006), EMBASE (1974 to Oct 2006), LILACS (1982 to Oct 2006), IBSS (1951 to Oct 2006) and Psychinfo (1996 to Oct 2006) using the search terms condom, contraceptive devices male, condom breakage, slippage and failure in combination with the Cochrane collaboration's search strategy for retrieving trials (figure 1).15 We searched conference proceedings, contacted researchers and organisations working in the field and checked the reference lists of all identified reports. Two reviewers independently scanned the electronic records to identify potentially trials.
Search strategy.
Data extraction
Two reviewers independently extracted data on the generation of the allocation sequence, allocation concealment, blinding and loss to follow-up according to the quality criteria developed by Juni9 (see key to additional table 1 online for full details). Overall losses to follow-up of up to 10% were scored adequate. We extracted data on the measure of effect used in each trial. In trials that collected short and long-term follow-up data, we extracted the long-term follow-up data. A reviewer contacted trial authors asking for all unclear or unreported methods and data. All discrepancies were agreed by discussion with a third reviewer. Trials that scored adequate for reporting of all four quality criteria were categorised as ‘high quality trials’. Data were extracted regarding whether clustering had been taken into account in the analysis.
Data analysis and synthesis
All analyses were conducted in STATA version 9.0. We used funnel plots to explore small study effects. We calculated the log of the ORs and standard mean differences (SMDs).16 For the purposes of meta-analysis, condom use outcomes during vaginal sex and unspecified type of sex were treated as the same outcome. Where two or more intervention arms were compared against a single control arm and the arms tested similar interventions, the most intensive intervention (with most components or longest duration) was included in the analysis. Where two or more diverse intervention arms were compared against a single control arm, or a factorial design was used, results are presented separately.
Poor trial quality is a source of bias so we report the results of trials that met the four quality criteria (allocation sequence, allocation concealment, loss to follow-up and blinding of outcome assessment) separately to other trials. We used random effects meta-analysis to give pooled estimates.17 Cluster randomised trial effect estimates were calculated based on the intra-cluster correlation co-efficient reported or, when not reported, the lowest of the published intra cluster co-efficient in the review.18 We examined heterogeneity visually by examining forest plots and statistically using the a χ2 test and I2 test for consistency.19 We explore the role of study quality via allocation concealment and inadequate or unclear blinding as these elements of study quality have been shown to influence outcomes reported.9
Results
The combined search strategies identified 622 electronic records. These were screened for eligibility and the full texts of 269 potentially eligible reports were obtained for further assessment. Out of the 269 potentially eligible reports, 138 reports containing 139 RCTs met the study inclusion criteria (figure 2). See additional table 1 online for a short description of all studies and the results of the quality assessment.
Associations of the effects of behavioural interventions on primary outcomes sexually transmitted infection (STI) and self-reported pregnancy.
Characteristics of studies
The 139 trials included approximately 143 000 participants. Of the 139 trials, one used a crossover design, 32 were cluster randomised trials and one used a factorial design. Altogether, 21 of the 32 cluster randomised trials reported having adjusted the results for clustering. Trial participants were recruited from several different settings, including healthcare (57 trials), education (28 trials), community (43 trials), military (1 trial) and unspecified (10 trials). Thirty-three trials had two or more intervention arms. The target populations were young people (48 trials), people with an STI (26 trials), intravenous drug users (19 trials), men who have sex with men (15 trials), other high risk individuals (17 trials), psychiatric patients (5 trials) and unspecified (15 trials). Altogether, 13 trials recruited participants from specific ethnic groups; the other 126 trials did not specify the ethnicity of participants. The median interval between randomisation and last outcome measurement was 26 weeks (IQR 13–52).
Interventions
The trials evaluated 181 different interventions. These were individual sexual behaviour change (n=156), sexual and intravenous drug behavioural change (n=19) and condom design (n=6). There were 23 simple interventions (with one or two components) and 158 complex interventions (with three or more components). The sexual behaviour change interventions addressed information, attitudes, condom use skills and/or condom availability, interpersonal factors within the sexual relationship influencing condom use and social factors influencing sex and condom use (see additional table 2 online). Sexual and intravenous drug behaviour interventions addressed safer injecting behaviour (15 trials), links between substance use and condom use (11 trials), substance use reduction (12 trials) and detoxification treatment (1 trial). Condom design trial interventions included providing a choice of condoms of different designs, different standards for manufacture of condoms, thicker/thinner condoms and different shapes of condom (baggy/straight shafted).
Outcomes and reporting bias
The trials included 90 different STI, pregnancy or condom use outcome measures. Trials reported between 1 and 49 outcomes per trial (median 11; IQR 7–17). Among the outcome measures used most frequently, 10 trials (7%) used the outcome ‘any STI’, 4 (3%) self-reported pregnancy and 22 (16%) used ‘condom use at last sex’.
Few trials used objective measures. Only 21(15%) trials reported a pregnancy or objective STI outcome measure. One trial used an objective measure of condom use.
Fifty-two trials did not provide enough data to calculate effect estimates so it was only possible to calculate effect estimates for 63% (n=87) of the trials.
Study quality
Only four trials scored adequate for reporting of all four quality criteria (allocation sequence, allocation concealment, loss to follow-up and blinding).31 35–37 The generation of the allocation sequence was adequate in 54 trials (39%), allocation concealment was adequate in 32 trials (23 %), losses to follow-up were adequate in 24 trials (17%) and outcome assessment was blinded in 34 trials (24%).
Effectiveness
For each type of intervention—sexual behaviour change interventions, sexual and intravenous drug behaviour change interventions and condom design interventions—we report the primary (pregnancy and STI) and secondary (condom use) outcomes. The results of high-quality trials are presented first followed by the results of other trials.
Sexual behaviour change interventions
Primary outcomes: pregnancy and STI
High-quality trial results
There was one trial which met all four quality criteria. Feldblum et al's trial evaluated peer education combined with individual risk counselling by a clinician among sex workers in Madagascar and reported a reduction in self-reported sexually transmitted disease symptoms OR=0.67 (0.51–0.89).35
Other trial results
Figure 3 shows forest plots for the effect of complex sexual behaviour change interventions on primary outcome measures. Three of the four trials reporting results regarding self-reported pregnancy had fewer pregnancies in the intervention group but no results were statistically significant. In 7 of the 10 trials reporting the outcome ‘any STI’ there were fewer STIs in the intervention group with five statistically significant results. One trial reported a statistically significant increase in ‘any STI’. Table 1 shows the effect estimates for trials reporting other STI outcomes. In 10 out of the 16 reported outcomes there were fewer STIs in the intervention group with three statistically significant results. One trial reported a statistically significant reduction in gonorrhoea and a statistically significant increase in syphilis.42
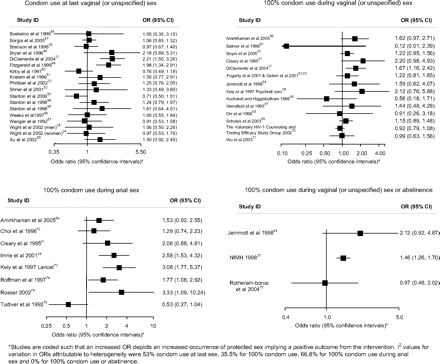
Associations of the effects of behavioural interventions on secondary binary outcomes measuring condom use during sex.
Primary outcomes for sexual behaviour change interventions
Exploring heterogeneity in STI outcomes according to study quality
Pooled estimates for the outcome ‘any STI’ 0.79 (95% CI 0.66 to 0.94) showed considerable heterogeneity (I2 64%, p=0.003). The pooled OR for ‘any STI’ among trials with adequate allocation concealment was 0.98 (95% CI 0.64 to 1.52; I2 75.9%; p=0.006) and for trials with inadequate or unclear allocation concealment was 0.73 (95% CI 0.64 to 0.83; I2 9.8%; p=0.353). The pooled OR for ‘any STI’ among trials with adequate blinding of the outcome assessor was 0.83 (95% CI 0.62 to 1.10; I2 73.4%; p=0.01) and for trials with inadequate or unclear blinding was 0.76 (95% CI 0.58 to 0.99; I2 39.1%; p=0.145).
Secondary outcomes: condom use
High-quality trial results
There were two trials that met all four quality criteria. Egger et al used an objective measure of condom use (finding a used condom in the motel room bin).44 They found that giving out condoms and providing condoms in motel rooms used for commercial sex increased condom use compared to having condoms available on request from reception (OR 1.32, 95% CI 1.03 to 1.61 and OR 1.31, 1.09 to 1.75, respectively. In motel rooms used for non-commercial sex, the same strategies also increased condom use for handing out condoms (OR 1.81, 95% CI 1.14 to 2.81) and for having condoms in motel rooms (OR 1.52, 95% CI 1.01 to 2.38). Providing health educational materials reduced condom use in commercial sex compared to when health educational materials were not provided (OR 0.89, 95% CI 0.84 to 0.94).44 Ehrhardt et al evaluated small group gender-specific discussion and used self-reported outcomes of either maintaining or improving safe sex (OR 1.64, 95% CI 0.95 to 2.86).45
Other trial results
Figures 4 and 5 and table 2 show the effect estimates for trials for each measure of condom use. For condom use at last sex, 9 of the 18 trials reported increases in condom use with two statistically significant results. For 100% condom use in vaginal (or unspecified) sex, 9 of the 14 trials reported increases in condom use although none was statistically significant. For 100% condom use for anal sex, seven of the eight trials reported increases in condom use with three statistically significant results. Two out of three trials reported increases in 100% condom use or abstinence with one statistically significant result. No trials reported increases in the frequency of protected sex. Three out of six trials reported statistically significant increases in the proportion of sex protected. For outcomes using condom use scales, two out of four trials reported increases in condom use of which one was statistically significant. One of three trials reported a statistically significant increase in the frequency of condom use. In 16 of the 22 other condom use outcomes reported, there was more condom use in the intervention group with eight showing statistical significance (table 2).
Associations of the effects of behavioural interventions on continuous secondary outcomes looking at frequency or proportion of unprotected sex or condom use.
Flow chart of systematic review.
Secondary outcomes for sexual behaviour change interventions
Sexual and intravenous drug behaviour change interventions
Primary outcomes: pregnancy and STI
High-quality trial results
There were no trials of sexual and intravenous drug behaviour change interventions that met all four quality criteria.
Other trial results
The Iguchi 1996 trial95 compared a 90-day drug detoxification programme to a 21-day drug detoxification programme and reported an OR consistent with a reduction in HIV acquisition (OR 0.37, 95% CI 0.11 to 1.28) (table 3).
Primary and secondary outcomes for sexual and intravenous drug behaviour change interventions
Secondary outcomes: condom use
High-quality trial results
There were no trials of sexual and intravenous drug behaviour change interventions reporting condom use outcomes that met all four quality criteria.
Other trial results
In 9 of the 13 condom use outcomes reported there was more condom use in the intervention group with three showing statistical significance (table 3).
Condom design interventions
Primary outcomes: pregnancy and STI
There were no trials of condom design interventions reporting primary (pregnancy or STI) outcomes.
Secondary outcomes: condom use
High-quality trial results
Golombok et al compared thicker condoms to thinner condoms and found that there was no difference in condom failure before or during sex (OR 1.06, 95% CI 0.79 to 1.41) (table 4).103
Primary and secondary outcomes for condom design interventions
Other trial results
The Steiner 2006 trial compared providing participants with a choice of different types of condom to providing one type of condom and reported a OR consistent with an increase in acquisition of ‘any STI’ (OR 1.31, 95% CI 0.80 to 2.15) for the ‘choice of condom’ arm.104 The Benton 1997 trial105 reported that Swiss quality seal standard condoms were less likely to break during anal sex than Australian standard condoms, and the Renzi 2003 trial106 of the reality female condom for anal sex reported this was less likely to slip during anal sex than a standard condom (table 4).
Discussion
Summary of findings
This review included over 143 000 study participants from 139 trials promoting effective condom use. Despite these research efforts, this review cannot provide reliable estimates of the effectiveness of interventions in promoting condom use due to the high potential for bias in the effect estimates.
The potential for bias is high for three main reasons. First, trials were of low quality and only four trials met all the quality criteria. Effect estimates have been found to be higher in lower quality studies where there is no allocation concealment and the results of our subgroup analysis according to allocation concealment are consistent with this.9 Second, most trials relied only on self-reported condom use outcomes (85%). Only one of the trials meeting all four quality criteria also used an objective outcome measure. Third, a low proportion of trials reported data using the same outcomes measure. Among the most commonly used outcomes, only 10 trials (7%) reported data regarding the outcome ‘any STI’, 4 (3%) reported outcome data for pregnancy and 22 (16%) reported outcome data for ‘condom use at last sex’. This is likely to have resulted in an overestimate of effects due to selective reporting of outcomes where statistically significant benefit is found. Thus, while the results reported in the trials in this review are generally consistent with modest benefits, the effect estimates cannot be considered reliable.
In the entire review there was only one trial that met all the quality criteria and used a single objective condom use outcome measure.35 Furthermore, the intervention was unique and, thus, there is no potential for selective reporting of outcomes in other similar trials. This trial demonstrated that either giving condoms to clients in motels or providing them in motel rooms was effective in increasing condom use for commercial and non-commercial sex.
Strengths and limitations of the review
This is the first comprehensive systematic review and methodological appraisal of all interventions promoting effective condom use. Descriptions of the intervention components are limited as these were based on the trial reports, which varied considerably in detail. Our analysis of trial quality as a source of heterogeneity, according to blinding and allocation concealment, for the outcome ‘any STI’ has limited power as few trials contributed to this pooled analysis. The heterogeneity of effect estimates means it is more appropriate to view individual study results; pooled estimates according to study quality are presented to show that these findings are consistent with earlier studies.9 We had insufficient power to explore any other aspects of trial quality as sources of heterogeneity. Among the trials reporting an increase in ‘any STI’, both the Imrie et al24 and Boyer et al21 trials had adequate allocation concealment. Other potential sources of heterogeneity include the type of participants and features of the intervention such as the duration, components and educational media used in the interventions. For example, among the trials reporting an increase in ‘any STI’, Imrie et al recruited men who defined themselves as ‘gay’24 and Carey et al recruited patients attending outpatient psychiatric care.22 Imrie et al's intervention was the only trial that was a single session intervention.24 Many of the components addressed in trials of interventions reporting increases in ‘any STI’ were similar to those addressed in the interventions reporting beneficial effects (eg, information, attitudes, self-efficacy, condom use skills, condom negotiation skills, motivation),21 22 24 but Imrie et al's intervention did not involve personal risk assessment, which was addressed by five of the trials reporting beneficial effects.24 The interventions also used different educational methods. For example, neither Imrie et al nor Carey et al used videos, which were used in six of the interventions reporting beneficial effects.22 24 We did not have sufficient power to robustly explore the type of participants and the duration, components and educational media used in the interventions as sources of heterogeneity in this systematic review.
Sources of bias in the systematic review
First, low trial quality in this review is an important potential source of bias. Effect estimates have been found to be higher where there is no allocation concealment and the results of our subgroup analysis according to allocation concealment are consistent with this.9 Second, the use of self-reported condom use outcomes is likely to have resulted in bias. Interventions promoting sexual behaviour change may influence reporting regarding behaviour more than actual behaviour and where participants are not blind to the intervention there may be differential misreporting of outcomes between the intervention and control group. Third, a low proportion of trials reported sufficient data to calculate effect estimates using the same outcome measures. Of the most commonly reported outcomes, only ten trials (10%) reported data regarding the outcome ‘any STI’, four (4%) reported the outcome ‘pregnancy’ and 22 (16%) reported condom use at last sex outcomes. Furukawa et al conducted an analysis of Cochrane reviews in which a median of 46% of trials (IQR 20–75%) reported sufficient data to calculate effect estimates using the same outcome.13 They found that in systematic reviews where a low proportion of trials used the same outcomes the effect estimates were higher than in systematic reviews where a high proportion of trials used the same outcomes. This is caused by selective reporting of outcomes in trials where no statistically significant benefit is found.13 Therefore, the low proportion of trials using the same outcome measures in this systematic review is likely to have resulted in an over-estimate of effect estimates.
Implications for research
Standards of conduct and reporting for trials promoting effective condom use must urgently be agreed. Consensus must be reached regarding which outcomes must be included irrespective of other reported outcomes. All future trials must include an objective measure of STI or pregnancy so that the trial results can be meaningfully compared and, where relevant, can contribute to future meta-analyses of objective biological outcomes. The components of interventions should be clearly described within trial reports. Trial protocols must be registered in advance with clearly specified outcomes. Trials promoting effective condom use should follow the existing guidance for the reporting and conduct of RCTS.108
Implications for condom promotion interventions
Condom distribution proximal to the time of sex has been shown to increase condom use in one high-quality trial in one setting. Innovative alternate means of distributing condoms proximal to the time of sex, especially among high-risk groups, should be evaluated. A high-quality trial of the female reality condom for anal sex should be conducted as results from a low-quality trial suggest the female reality condom may be less likely to slip than a standard condom. Future sexual behaviour change interventions should be based on the content of existing interventions that report beneficial effects. Such interventions should be evaluated by an adequately powered high-quality RCT.
Conclusion
Increasing effective condom use is of global public health significance. Reported results in the trials in this review are generally consistent with modest benefits, but bias introduced by the poor quality of trials, reliance on self-reported outcomes and selective reporting of outcomes mean that the reported results are likely to be an over-estimate of effects. Robust conclusions regarding the effectiveness of interventions promoting effective condom use cannot be made. Future trials promoting effective condom use must be conducted and reported to the highest standards.
What is already known
CONSORT standards for the conduct and reporting of trials are well-established.
What this paper adds
Despite the public health importance of increasing condom use, there is little reliable evidence on the effectiveness of condom promotion interventions.
There is considerable potential for bias in trials of interventions promoting effective condom use due to poor trial quality.
Because of the low proportion of trials using the same outcome, the potential for bias from selective reporting of outcomes is also considerable.
Standards of conduct and reporting for trials promoting effective condom use must be agreed and consensus must be reached regarding which outcomes should be reported in all trials irrespective of other reported outcomes.
References
Footnotes
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.