Abstract
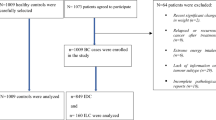
A case-cohort analysis of the association between diet and risk of benign proliferative epithelial disorders (BPED) of the breast was undertaken within a cohort of 56,537 women who were enrolled in the Canadian National Breast Screening Study (NBSS) and who completed a self-administered dietary questionnaire. (The NBSS is a randomized controlled trial of screening for breast cancer in women aged 40 to 59 years.) BPED are thought to have premalignant potential. Specific hypotheses were that risk of BPED would increase with increasing energy-adjusted fat intake and decrease with increasing energy-adjusted vitamin A and fiber intake. Additionally, we explored the association between calcium intake and risk of BPED. During the active follow-up phase of the NBSS, 657 women in the dietary cohort were diagnosed with biopsy-confirmed incident BPED. For comparative purposes, a subcohort consisting of a random sample of 5,581 women was selected from the full dietary cohort. After exclusions for various reasons, the analyses were based on 545 cases and 4,921 non-cases. Overall, the results were almost uniformly null, and provided little support for the study hypotheses. Rate ratios (95 percent confidence intervals [CI]) for the highest cf the lowest quintile levels for total fat, retinol, β-carotene, fiber, and calcium were 0.88 (CI = 0.65-1.20), 0.97 (CI = 0.71-1.31), 0.94 (CI = 0.70-1.27), 1.11 (CI = 0.82-1.50), and 0.81 (CI = 0.60-1.07), respectively. There were too few cases of atypical BPED for meaningful analysis, but results for those whose BPED showed no atypia were similar to the overall results. Further analyses conducted separately in the screened and control arms of the NBSS also failed to provide strong support for dietary associations, as did those conducted separately for screen-detected and interval-detected BPED.
Similar content being viewed by others
References
Wynder EL, Cohen LA, Muscat JE, Winters B, Dwyer JT, Blackburn G. Breast cancer: weighing the evidence for a promoting role of dietary fat. J Natl Cancer Inst1997; 89: 766–75.
Hunter DJ, Spiegelman D, Adami HO, et al. Cohort studies of fat intake and the risk of breast cancer - a pooled analysis. N Engl J Med1996; 334: 356–61.
Willett WC, Stampfer MJ, Colditz GA, Rosner GA, Hennekens CH, Speizer FE. Dietary fat and the risk of breast cancer. N Engl J Med1987; 316: 22–8.
Wang DY, Fentiman IS. Epidemiology and endocrinology of benign breast disease. Breast Cancer Res Treat1985; 6: 5–36.
Zhang I, Bird RP, Bruce WR. Proliferative activity of murine mammary epithelium as affected by dietary fat and calcium. Cancer Res1987; 47: 4905–8.
Jacobsen EA, Russell R, Newmark HL, et al. Fat, calcium, and tumor development in dimethylbenz(a)anthracene (DMBA)-treated rats. Proc Can Fed Biol Soc1987; 30: 112.
Miller AB, Howe GR, Wall C. The national study of breast cancer screening: protocol for a Canadian randomized controlled trial of screening for breast cancer in women. Clin Invest Med1981; 4: 227–58.
Miller AB, Baines CJ, To T, Wall C. Canadian National Breast Screening Study. I. Breast cancer detection and death rates among women aged 40 to 49 years. II. Breast cancer detection and death rates among women aged 50 to 59 years. CMAJ1992; 147: 1459–88.
Jain MG, Howe GR, Johnson KC, Miller AB. Evaluation of a diet history questionnaire for epidemiologic studies. Am J Epidemiol1980; 111: 212–9.
Morgan RW, Jain M, Miller AB, et al. A comparison of dietary methods in epidemiologic studies. Am J Epidemiol1978; 107: 488–98.
Jain MG, Harrison L, Howe GR, et al. Evaluation of a self-administered dietary questionnaire for use in a cohort study. Am J Clin Nutr1982; 36: 931–5.
Robles SC, Marrett LD, Clarke EA, et al. An application of capture-recapture methods to the estimation of completeness of cancer registration. J Clin Epidemiol1988; 41: 495–501.
Page DL, Anderson TJ. Diagnostic Histopathology of the Breast. Edinburgh, Scotland (UK): Churchill Livingstone, 1987.
Huber PJ. The behavior of maximum likelihood estimates under non-standard conditions. Berkeley, California (USA): University of California Press, 1967; Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability, Vol. 1: 221–33.
Willett WC, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol1986; 124: 17–27.
Kipnis V, Freedman L, Brown CC, et al. Interpretation of energy adjustment methods for nutritional epidemiology. Am J Epidemiol1993; 137: 1376–80.
Dubin N, Pasternack BS. Breast cancer screening data in case-control studies. Am J Epidemiol1984; 120: 8–16.
Rimm EB, Giovannucci EL, Stampfer MJ, et al. Reproducibility and validity of an expanded self-administered semiquantitative food-frequency questionnaire among male health professionals. Am J Epidemiol1992; 135: 1114–26.
Rosner B, Willett WC, Spiegelman D. Correction of logistic regression relative risk estimates and confidence intervals for systematic within-person measurement error. Stat Med1989; 8: 1051–69.
Lubin F, Wax Y, Ron E, et al. Nutritional factors associated with benign breast disease etiology: a case-control study. Am J Clin Nutr1989; 50: 551–6.
Hislop TG, Band PR, Deschamps M, et al. Diet and histologic types of benign breast disease defined by subsequent risk of breast cancer. Am J Epidemiol1990; 131: 263–70.
Rohan TE, Cook MG, Potter JD, McMichael AJ. A case-control study of diet and benign proliferative epithelial disorders of the breast. Cancer Res1990; 50: 3176–81.
Ingram DM, Nottage E, Roberts T. The role of diet in the development of breast cancer: a case-control study of patients with breast cancer, benign epithelial hyperplasia and fibrocystic disease of the breast. Br J Cancer1991; 64: 187–91.
London SJ, Sacks FM, Stampfer MJ, et al. Fatty acid composition of the subcutaneous adipose tissue and risk of proliferative benign breast disease and breast cancer. J Natl Cancer Inst1993; 85: 785–93.
Baghurst PA, Rohan TE. Dietary fibre and risk of benign proliferative epithelial disorders of the breast. Int J Cancer1995; 63: 481–5.
London SJ, Stein EA, Henderson IC, et al. Carotenoids, retinol, and vitamin E and risk of proliferative benign breast disease and breast cancer. Cancer Causes Control1992; 3: 503–12.
Rose DP, Boyar AP, Cohen C, Strong LE. Effect of a low-fat diet on hormone levels in women with cystic breast disease. I. Serum steroids and gonadotrophins. J Natl Cancer Inst1987; 78: 623–6.
Welsch CW. Environmental enhancement of mammary tumorigenesis by dietary fat: review of potential mechanisms. Am J Clin Nutr1987; 45: 192–202.
Benson J, Lev M, Grand CG. Enhancement of mammary fibroadenomas in the female rat by a high fat diet. Cancer Res1956; 16: 135–7.
Khan N, Yang K, Newmark H, et al. Mammary ductal epithelial cell hyperproliferation and hyperplasia induced by a nutritional stress diet containing four components of a Western-style diet. Carcinogenesis1994; 15: 2645–8.
Kidwell WR, Knazek RA, Vonderhaar BK, Losonczy I. Effect of unsaturated fatty acids on the development and proliferation of normal and neoplastic breast epithelium. In: Arnott MS, van Eys J, Wang YM, eds. Molecular Interrelations of Nutrition and Cancer. New York, NY (USA): Raven Press, 1982: 219–36.
Balakrishnan A, Cramer S, Bandyopadhay GK, et al. Differential proliferative response to linoleate in cultures of epithelial cells from normal human breast and fibroadenomas. Cancer Res1989; 49: 857–62.
Schultz TD, Howie BJ. In vitrobinding of steroid hormones by natural and purified fibers. Nutr Cancer1986; 8: 141–7.
Adlercreutz H, Fotsis T, Bannwart C, et al. Determination of urinary lignans and phytoestrogen metabolites, potential anti-estrogens and anti-carcinogens, in urine of women on various habitual diets. J Steroid Biochem1986; 25: 791–7.
Goldin BR, Woods MN, Spiegelman DL, et al. The effect of dietary fat and fiber on serum estrogen concentrations in premenopausal women under controlled dietary conditions. Cancer1994; 74: 1125–31.
Schaefer EJ, Lamon-Fava S, Spiegelman D, et al. Changes in plasma lipoprotein concentrations and composition in response to a low-fat, high-fiber diet are associated with changes in serum estrogen concentrations in premenopausal women. Metabolism1995; 44: 749–56.
Sporn MB, Roberts AB. Role of retinoids in differentiation and carcinogenesis. Cancer Res1983; 43: 3034–40.
Wang XD. Review: absorption and metabolism of betacarotene. J Am Coll Nutr1994; 13: 314–25.
Krinsky NI. Actions of carotenoids in biological systems. Ann Rev Nutr1993; 13: 561–87.
Potter JD. Nutrition and colorectal cancer. Cancer Causes Control1996; 7: 127–46.
Swierenga SH, Whitfield JF, Boynton AL, et al. Regulation of proliferation of normal and neoplastic rat liver cells by calcium and cyclic AMP. Ann NY Acad Sci1980; 349: 294–311.
Bodian CA. Benign breast diseases, carcinoma in situ, and breast cancer risk. Epidemiol Rev1993; 15: 177–87.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rohan, T.E., Jain, M. & Miller, A.B. A case-cohort study of diet and risk of benign proliferative epithelial disorders of the breast (Canada). Cancer Causes Control 9, 19–27 (1998). https://doi.org/10.1023/A:1008841118358
Issue Date:
DOI: https://doi.org/10.1023/A:1008841118358