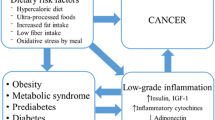
Some factors related to Westernization or industrialization increase risk of colon cancer. It is believed widely that this increase in risk is related to the direct effects of dietary fat and fiber in the colonic lumen. However, the fat and fiber hypotheses, at least as originally formulated, do not explain adequately many emerging findings from recent epidemiologic studies. An alternative hypothesis, that hyperinsulinemia promotes colon carcinogenesis, is presented here. Insulin is an important growth factor of colonic epithelial cells and is a mitogen of tumor cell growth in vitro. Epidemiologic evidence supporting the insulin/colon-cancer hypothesis is largely indirect and based on the similarity of factors which produce elevated insulin levels with those related to colon cancer risk. Specifically, obesity—particularly central obesity, physical inactivity, and possibly a low dietary polyunsaturated fat to saturated fat ratio—are major determinants of insulin resistance and hyperinsulinemia, and appear related to colon cancer risk. Moreover, a diet high in refined carbohydrates and low in water-soluble fiber, which is associated with an increased risk of colon cancer, causes rapid intestinal absorption of glucose into the blood leading to postprandial hyperinsulinemia. The combination of insulin resistance and high glycemic load produces particularly high insulin levels. Thus, hyperinsulinemia may explain why obesity, physical inactivity, and a diet low in fruits and vegetables and high in red meat and extensively processed foods, all common in the West, increase colon cancer risk.
Similar content being viewed by others
References
Haenszel W, Kurihara M. Studies of Japanese migrants. I. Mortality from cancer and other diseases among Japanese in the United States. JNCI 1968; 40: 43–68.
Boyle P, Zaridze DG, Smans M. Descriptive epidemiology of colorectal cancer. Int J Cancer 1985; 36: 9–18.
Cannon-Allbright LA, Skolnick MH, Bishop DT, Lee RG, Burt RW. Common inheritance of susceptibility to colonic adenomatous polyps and associated colorectal cancers. N Engl J Med 1988; 319: 533–7.
Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med 1988; 319: 525–32.
Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer:in the United States today. JNCI 1981; 66: 1191–308.
Willett WC. The search for the causes of breast and colon cancer. Nature 1989; 338: 389–94.
Armstrong B, Doll R. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int J Cancer 1975; 15: 617–31.
Rose DP, Boyer AP, Wynder EL. International comparisons of mortality rates for cancer of the breast, ovary, prostrate, and colon, and per capita food consumption. Cancer 1986; 58: 2363–71.
Phillips RL, Garfinkel L, Kuzma JW, Beeson WL, Lotz T, Brin B. Mortality among California Seventh-Day Adventists for selected cancer sites. JNCI 1980; 65: 1097–107.
Hill MJ. The role of unsaturated bile acids in the etiology of large bowel cancer. In: Hiatt HH, Watson JD, Winsten JA, eds. Origins of Human Cancer. Cold Spring Harbor, NY (USA): Cold Spring Harbor Laboratory, 1977: 1627–40.
Reddy BS. Diet and excretion of bile acids. Cancer Res 1981; 41: 3766–8.
Chomchai C, Bhadrachari N, Nigro ND. The effect of bile on the induction of experimental intestinal tumors in rats. Dis Colon Rectum 1974; 17: 310–2.
Narisawa T, Magadia NE, Weisburger JH, Wynder EL. Promoting effects of bile acids on colon carcinogenesis after intrarectal instillation of N-methyl-N-nitro-N-nitrosoquanidine in rats. NNCI 1974; 53: 1093–7.
Burkitt DP. Epidemiology of cancer of the colon and rectum. Cancer 1971; 28: 3–13.
Liu K, Stamler J, Moss D, Garside D, Persky V, Soltero I. Dietary cholesterol, fat and fibre and colon cancer mortality. An analysis of international data. Lancet 1979; ii: 782–5.
Vena JE, Graham S, Zielezny M, et al. Occupational exercise and risk of cancer. Am J Clin Nutr 1987; 45: 318–27.
Peters RK, Garabrant DH, Yu MC, et al. A case-control study of occupational and dietary factors in colorectal cancer in young men by subsite. Cancer Res 1989; 49: 5459–68.
Garabrant DH, Peters JM, Mack TM, et al. Job activity and colon cancer risk. Am J Epidemiol 1984; 119: 1005–14.
Vena JE, Graham S, Zielezny M, et al. Lifetime occupational exercise and colon cancer. Am J Epidemiol 1985; 122: 357–65.
Brownson RC, Zahm SH, Change JC, et al. Occupational risk of colon cancer. An analysis by anatomic subsite. Am J Epidemiol 1989; 130: 675–87.
Fredriksson M, Bengtsson NO, Hardell L, et al. Colon cancer, physical activity, and occupational exposures. A case-control study. Cancer 1989; 63: 1838–42.
Gerhardsson M, Norell SE, Kiviranta H, et al. Sedentary jobs and colon cancer risk. Am J Epidemiol 1986; 123: 775–80.
Paffenbarger RSJr, Hyde RT, Wing AL. Physical activity and incidence of cancer in diverse populations: A preliminary report. Am J Clin Nutr 1987; 45: 312–7.
Fraser G, Pearce N. Occupational physical activity and risk of cancer of the colon and rectum in New Zealand males. Cancer Causes Control 1993; 4: 45–50.
Wu AH, Paganini-Hill A, Ross RK, et al. Alcohol, physical activity, and other risk factors for colorectal cancer: A prospective study. Br J Cancer 1987; 55: 687–94.
Polednak AP. College athletics, body size, and cancer mortality. Cancer 1976; 38: 382–7.
Slattery ML, Schumacher MC, Smith KR, et al. Physical activity, diet and risk of colon cancer in Utah. Am J Epidemiol 1988; 128: 989–99.
Gerhardsson M, Floderus B, Norell SE. Physical activity and colon cancer risk. Int J Epidemiol 1988; 7: 743–6.
Gerhardsson de Verdier M, Steineck G, Hagman U, et al. Physical activity and colon cancer: A case-referent study in Stockholm. Int J Cancer 1990; 46: 985–9.
Severson RK, Nomura AMY, Grove JS, Stemmermann GN. A prospective analysis of physical activity and cancer. Am J Epidemiol 1989; 130: 522–9.
Albanes D, Blair A, Taylor PR. Physical activity and risk of cancer in the NHANES I population. Am J Public Health 1989; 79: 744–50.
Ballard-Barbash R, Schatzkin A, Albanes D, et al. Physical activity and risk of large bowel cancer in the Framingham Study. Cancer Res 1990; 50: 3610–3.
Kato I, Tominaga S, Matsuura A, Yoshii Y, Shirai M, Kobayashi S. A comparative case-control study of colorectal cancer and adenoma. Jpn J Cancer Res 1990; 81: 1101–8.
Whittemore AS, Wu-Williams AH, Lee M, et al. Diet, physical activity, and colorectal cancer among Chinese in North America and China. JNCI 1990; 82: 915–26.
Lee IM, Paffenbarger RS. Quetelet's index and risk of colon cancer in college alumni. JNCI 1992; 84: 1326–31.
Neugut AI, Lee WC, Garbowski GC, et al. Obesity and colorectal adenomatous polyps. JNCI 1991; 83: 359–61.
Little J, Logan RFA, Hawtin PG, Hardcastle JD, Turner ID. Colorectal adenomas and energy intake, body size and physical activity: a case-control study of subjects participating in the Nottingham faecal occult blood screening programme. Br J Cancer 1993; 67: 177–84.
Kono S, Shinchi K, Ikeda N, Yanai F, Imanishi K. Physical activity, dietary habits and adenomatous polyps of the sigmoid colon: A study of self-defense officials in Japan. J Clin Epidemiol 1991; 44: 1255–61.
Bayerdorffer E, Mannes GA, Oshsenkuhn, Kopcke W, Wiebecke B, Paumgartner G. Increased risk of ‘highrisk’ colorectal adenomas in overweight men. Gastroenterol 1993; 104: 137–44.
Lew EA, Garfinkel L. Variations in mortality by weight among 750,000 men and women. J Chron Dis 1979; 32: 563–76.
Waaler HT. Height, weight and mortality: the Norwegian experience. Acta Med Scand Suppl 1984; 679: 1–56.
Phillips RL, Snowden DA. Dietary relationships with fatal colorectal cancer among Seventh-Day Adventists. JNCI 1985; 74: 307–17.
Garland C, Barrett-Conner E, Rossof AH, et al. Dietary vitamin D and calcium and risk of colorectal cancer: A 19 year prospective study in men. Lance 1985; i: 307–9.
Klatsky AL, Armstrong MA, Friedman GD, Hiatt RA. The relations of alcoholic beverage use to colon and rectal cancer. Am J Epidemiol 1988; 128: 1007–15.
Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med 1992; 37: 1350–5.
Le Marchand L, Wilkins LR, Mi MP. Obesity in youth and middle age and risk of colorectal cancer in men. Cancer Causes Control 1992; 3: 349–54.
Nomura A, Heilbrun LK, Stemmermann GN. Body mass index as a predictor of cancer in men. JNCI 1985; 74: 319–23.
Chute CG, Willett WC, Colditz GA, et al. A prospective study of body mass index, height, and smoking on risk of colorectal cancer in women. Cancer Causes Control 1991; 2: 117–24.
Kune GA, Kune S, Watson LF. Body weight and physical activity as predictors of colorectal cancer risk. Nutr Cancer 1990; 13: 9–17.
Graham S, Marshall J, Haughey B, et al. Dietary epidemiology of cancer of the colon in western New York. Am J Epidemiol 1988; 128: 490–503.
West DW, Slattery ML, Robison LM, Schuman KL, Ford MH, Mahoney AW, Lyon JL, Sorensen AW. Dietary intake and colon cancer: sex and anatomic sitespecific associations. Am J Epidemiol 1989; 130: 883–94.
Gerhardsson de Verdier M, Hagman U, Steineck G, et al. Diet, body mass index and colorectal cancer: A casereferent study in Stockholm. Int J Cancer 1990; 46: 832–8.
Bjelke E. Epidemiology of colorectal cancer, with emphasis on diet. In: Davis W, Harrup KR, Stathopoulos G, eds. Human Cancer. Its Characterization and Treatment. Amsterdam, Netherlands: Exerpta Medica, Int. Congress Series No. 484, 1980: 158–74.
Stemmermann GN, Nomura AM, Heilbrun LK. Dietary fat and the risk of colorectal cancer. Cancer Res 1984; 44: 4633–7.
Hirayama T. A large-scale study on cancer risks by diet-with special reference to the risk reducing effects of green-yellow vegetable consumption. In: Hayashi Y, Magao M, Sugimura T, et al., eds. Diet, Nutrition, and Cancer. Tokyo, Japan: Japan Scientific Societies Press, 1986: 41–53.
Garland C, Shekelle RE, Barrett-Connor E, Criqui MH, Rossof AH, Paul O. Dietary vitamin D and calcium and risk of colorectal cancer: a 19-year prospective study in men. Lancet 1985; 1: 307–9.
Phillips RL, Snowdon DA. Association of meat and coffee use with cancers of the large bowel, breast, and prostate among Seventh-day Adventists: Preliminary results. Cancer Res (Suppl) 1983; 43: 2403–8.
Willett WC, Stampfer MJ, Colditz GA, Rosner BA, Speizer FE. Relation of meat, fat, and fiber intake to the risk of colon cancer in a prospective study among women. N Engl J med 1990; 323: 1664–72.
Goldbohm RA, van den Brandt PA, van't Veer P, et al. A prospective cohort study on the relation between meat consumption and the risk of colon cancer. Cancer Res 1994; 54: 718–23.
Bostick RM, Potter JD, Kushi, et al. Sugar, meat, and fat intake, and non-dietary risk factors for colon cancer incidence in Iowa women (United States). Cancer Causes Control 1994; 5: 38–52.
Giovannucci E, Rimm EB, Stampfer MJ, et al. Intake of fat, meat and fiber in relation to risk of colon cancer in men. Cancer Res 1994; 54: 2390–7.
Haenszel W, Locke FB. Segi M. A case-control study of large bowel cancer in Japan. JNCI 1980; 64: 17–22.
Macquart-Moulin G, Riboli E, Cornee J, Charnay B, Berthezene P, Day N. Case-control study on colorectal cancer and diet in Marseilles. Int J Cancer 1986; 38: 183–91.
Berta JL, Coste T, Rautureau J, Guilloud-Bataille M, Pequignot G. Diet and rectocolonic cancers. Results of a case-control study. Gastroenterol Clin Biol 1985; 9: 348–53.
Tuyns AJ, Haelterman M, Kaaks R. Colorectal cancer and the intake of nutrients: oligosaccharides are a risk factor fats are not. A case-control study in Belgium. Nutr Cancer 1987; 10: 181–96.
Meyer F, White E. Alcohol and nutrients in relation to colon cancer in middle-aged adults. Am J Epidemiol 1993; 138: 225–36.
Jain M, Cook GM, Davis FG, Grace MG, Howe GR, Miller AB. A case-control study of diet and colo-rectal cancer. Int J Cancer 1980; 26: 757–68.
Potter JD, McMichael AJ. Diet and cancer of the colon and rectum: a case-control study. JNCI 1986; 76: 557–69.
Lyon JL, Mahoney AW, West DW, et al. Energy intake: its relationship to colon cancer risk. JNCI 1987; 78: 853–61.
Bristol JB, Emmett PM, Heaton KW, Williamson RC. Sugar, fat, and the risk of colorectal cancer. Br Med J 1985; 291: 1467–70.
Peters RK, Pike MC, Garabrant D, Mack TM. Diet and colon cancer in Los Angeles County, California. Cancer Causes Control 1992; 3: 457–73.
Haenszel W, Berg JW, Segi M, et al. Large bowel cancer in Hawaiian Japanese. JNCI 1973; 51: 1765–9.
Gerhardsoon de Verdier M, Hagman U, Peters RK, Steineck G. Meat, cooking methods and colorectal cancer: A case-referent study in Stockholm. Int J Cancer 1991; 49: 520–5.
Manousos O, Day NE, Trichopoulos D, Gerovassilis F, Tzonou A, Polychronopoulou A. Diet and colorectal cancer: a case-control study in Greece. Int J Cancer 1983; 32: 1–5.
La Vecchia C, Negri E, DeCarli A, et al. A case-control study of diet and colorectal cancer in Northern Italy. Int J Cancer 1988; 41: 492–8.
Miller AB, Howe GR, Jain M, Craib KJP, Harrison L. Food items and food groups as risk factors in a case-control study of diet and colorectal cancer. Int J Cancer 1983; 32: 155–61.
Young TB, Wolf TB. Case-control study of proximal and distal colon cancer and diet in Wisconsin. Int J Cancer 1988; 42: 167–75.
Benito E, Obrador A, Stiggelbout A, et al. A population-based case-control study of colorectal cancer in Majorca. Int J Cancer 1990; 45: 69–76.
Lee HP, Gourley L, Duffy SW, Esteve J, Lee J, Day NE. Colorectal cancer and diet in an Asian population-A case-control study among Singapore Chinese. Int J Cancer 1989; 43: 1007–16.
Gerhardsson de Verdier M, Hagman U, Peters RK, Steineck G. Meat cooking methods and colorectal cancer: A case-referent study in Stockholm. Int J Cancer 1991; 49: 520–5.
Kune S, Kune GA, Watson LF. Case-control study of dietary etiologic factors: the Melbourne Colorectal Cancer Study. Nutr Cancer 1987; 9: 21–42.
Steinmetz KA, Potter JD. Food-group consumption and colon cancer in the Adelaide Case-Control Study. II. Meat, poultry, seafood, dairy foods and eggs. Int J Cancer 1993; 53: 711–19.
Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 1986; 124: 17–27.
Turesky RJ, Lang N, Butler MA, et al. Metabolic activation of carcinogenic heterocyclic aromatic amines by human liver and colon. Carcinogenesis 1991; 12: 1417–21.
Modan B, Barell V, Lubin F, Modan M, Greenberg RA. Low fiber intake as an etiology factor in cancer of the colon. JNCI 1975; 55: 15–8.
Dales LC, Friedman GD, Ury HK, Grossman S, Williams SR. A case-control study of relationships of diet and other traits to colorectal cancer in American blacks. Am J Epidemiol 1979; 109: 132–44.
Iscovich JM, L'Abbe KA, Castelleto R, et al. Colon cancer in Argentina. II: Risk from fiber, fat and nutrients. Int J Cancer 1992; 51: 858–61.
Trock B, Lanza E, Greenwald P. Dietary fiber, vegetables, and colon cancer: critical review and meta-analyses of the epidemiologic evidence. JNCI 1990; 82: 650–61.
Thun MJ, Calle EE, Namboodiri MM, et al. Risk factors for fatal cancer in a large prospective study. JNCI 1992; 84: 1491–1500.
Heilbrun LK, Nomura A, Hankin JH, Stemmermann GN. Diet and colorectal cancer with special reference to fiber intake. Int J Cancer 1989; 44: 1–6.
Steinmetz KA, Kushi LH, Bostick RM, Folsom AR, Potter JD. Vegetables, fruit, and colon cancer in the Iowa Women's Study. Am J Epidemiol 1994; 139: 1–15.
Giovannucci E, Stampfer MJ, Colditz G, Rimm EB, Willett WC. Relationship of diet to risk of colorectal adenoma in men. JNCI 1992; 84: 91–8.
Neugut AI, Garbowski GC, Lee WC, et al. Dietary risk factors for the incidence and recurrence of colorectal adenomatous polyps. A case-control study. Ann Int Med 1993; 11: 91–5.
Hoff G, Moen IE, Trygg K, et al. Epidemiology of polyps in the rectum and sigmoid colon. Evaluation of nutritional factors. Scand J Gastroenterology 1986; 21: 199–204.
Sandler RS, Lyles CM, Peipins LA, McAuliffe CA, Woosley JT, Kupper LL. Diet and the risk of colorectal adenomas: macronutrients, cholesterol and fiber. JNCI 1993; 85: 875–84.
Benito E, Cabeza E, Moreno V, Obrador A, Bosch FX. Diet and colorectal adenomas: a case-control study in Majorca. Int J Cancer 1993; 55: 213–9.
Macquart-Moulin G, Riboli E, Cornee J, Kaaks R, Berthezene P. Colorectal polyps and diet: A case-control study in Marseilles. Int J Cancer 1987; 40: 179–88.
Phillips R. Role of life-style and dietary habits in risk of cancer among Seventh-day Adventists. Cancer Res 1975; 35: 3513–22.
Pickle LW, Greene MH, Ziegler RG, et al. Colorectal cancer in rural Nebraska. Cancer Res 1984; 44: 363–9.
Bidoli E, Franceschi S, Talamini R, Barra S, La Vecchia C. Food consumption and cancer of the colon and rectum in north-eastern Italy. Int J Cancer 1992; 50: 223–9.
Centonze S, Boeing H, Leoci C, Guerra V, Misciagna G. Dietary habits and colorectal cancer in a low-risk area. Results from a population-based case-control study in Southern Italy. Nutr Cancer 1994; 21: 233–46.
Weisburger JH. Mechanisms of macronutrient carcinogenesis. In: Micozzi MC, Moon TE, eds. Macronutrients: Investigating Their Role in Cancer New York, NY (USA): Marcel Dekker, Inc. 1992: 3–31.
Cummings JH, Branch W, Jenkins DJA, Southgate DAT, Houston H, James WPT. Colonic response to dietary fiber from carrot, cabbage, apple, bran and guar gum. Lancet 1978; 1: 5–9.
Lampe JW, Fredstrom SB, Slavin JL, Potter JD. Sex differences in colonic function: a randomised trial. Gut 1993; 34: 531–6.
Prentice RL, Kaker S, Hursting S, Sheppard L, Klein R, Kushi L. Aspects of the rationale for the Women's Health Trial. JNCI 1988; 80: 802–14.
Aaronson SA. Growth factors and cancer. Science 1991; 254: 1146–53.
Watkins LF, Lewis LR, Levine AE. Characterization of the synergistic effects of insulin and transferrin and the regulation of their receptors on a human colon carcinoma cell line. Int J Cancer 1990; 45: 372–5.
Koenuma M, Yamori, Tsuruo T. Insulin and insulin-like growth factor 1 stimulate proliferation of metastatic variants of colon carcinoma 26. Jpn J Cancer Res 1989; 80: 51–8.
Bjork J, Nilsson J, Hultcrantz R, Johansson C. Growth-regulatory effects of sensory neuropeptides, epidermal growth factor, insulin, and somatostatin on the nontransformed intestinal epithelial cell line IEC-6 and the colon cancer cell line HT 29. Scand J Gastroenterol 1993; 28: 879–84.
MacDonald RS, Thornton WH, Bean TL. Insulin and IGE-1 receptors in a human intestinal adenocarcinoma cell line (CACO-2): regulation of NA+ glucose transport across the brush border. J Receptor Res 1993; 13: 1093–113.
Guo YS, Narayan S, Yallampalli C, Singh P. Characterization of insulin like growth factor I receptors in human colon cancer. Gastroenterology 1992; 102: 1101–8.
Macaulay VM. Insulin-like growth factors and cancer. Br J Cancer 1992; 65: 311–20.
Soos MA, Whittaker J, Lammers R, Ullrich A, Siddle K. Receptors for insulin and insulin-like growth factor-I can form hybrid dimers. Characterization of hybrid receptors in transfected cells. Biochem J 1990; 270: 383–90.
Bach LA, Rechler MM. Insulin-like growth factors and diabetes. Diabetes Metab Rev 1992; 8: 229–57.
Flier JS, Usher P, Moses AC. Monoclonal antibody to the type I insulin-like growth factor (IGF-I) receptor blocks IGF-I receptor-mediated DNA synthesis: clarification of the mitogenic mechanisms of IGF-I and insulin in skin fibroblasts. Proc Natl Acad Sci USA 1986; 93: 664–8.
Moller DE, Flier JS. Insulin resistance-mechanisms, syndromes, and implications. N Engl J Med 1991; 325: 938–48.
Papa V, Pezzino V, Costantino A, et al. Elevated insulin receptor content in human breast cancer. J Clin Invest 1990; 86: 1503–10.
Bruning PF, Bonfrer JMG, van Noord PAH, Hart AAM, de Jong-Dakker M. Insulin resistance and breast-cancer risk. Int J Cancer 1992; 52: 511–6.
Folsom AR, Kaye SA, Princeas RJ, Potter JD, Gapstur SM, Wallace RC. Increased incidence of carcinoma of the breast associated with abdominal adiposity in postmenopausal women. Am J Epidemiol 1990; 131: 794–803.
Klein I, Parveen G, Gavaler JS, Venthiel DH. Colonic polyps in patients with acromegaly. Ann Intern Med 1982; 97: 27–30.
Ituarte EM, Petrini J, Hershman JM. Acromegaly and colon cancer. Ann Intern Med 1984; 101: 627–8.
Pines A, Rozen P, Ron E, Gilat T. Gastrointestinal tumors in acromegalic patients. Am J Gastroenterol 1985; 80: 266–9.
Ritter MM, Richter WO, Schwandt P. Acromegaly and colon cancer. Ann Intern Med 1987; 106: 636–7.
Ziel FH, Peters AL. Acromegaly and gastrointestinal adenocarcinomas. Ann Intern Med 1988; 109: 514–5.
Brunner JE, Johnson CC, Zafer S, Peterson EL, Brunner JF, Mellinger RC. Colon cancer and polyps in acromegaly: increased risk associated with family history of colon cancer. Clin Endocrinol 1990; 32: 65–71.
Terzolo M, Tappero G, Borretta G, et al. High prevalence of colonic polyps in patients with acromegaly. Arch Intern Med 1994; 154: 1272–6.
Barzilay J, Heatley GJ, Cushing GW. Benign and malignant tumors in patients with acromegaly. Arch Intern Med 1991; 151: 1629–32.
Cats A, Dullaart RP, Zwart N, Kleibeuker JH, de Vries EG. Increased colonic epithelial cell proliferation in acromegalic patients (Meeting abstract). Proc Annu Meet Am Assoc Cancer Res 1993; 34: A1142..
Tricoli JV, Rall LB, Karakousis CP, Herrera L, Petrelli NJ, Bell GI. Enhance levels of insulin-like growth factor messenger RNA in human colon carcinomas and liposarcomas. Cancer Res 1986; 46: 6169–80.
Lambert S, Collette J, Gillis J, Franchimont P, Desaive C, Gol-Winkler R. Tumor IGF-II content in a patient with a colon adenocarcinoma correlates with abnormal expression of the gene. Int J Cancer 1991; 48: 826–30.
Culouscou JM, Remacle-Bonnet M, Garrouste VJ, Fantini J, Marvaldi J, Pommier G. Production of insulin-like growth factor II (IGF-II) and different forms of IGF-binding proteins by HT-29 human colon cancer cell line. J Cell Physiol 1990; 143: 405–15.
Boudewijn M, Burgering BM, Medema RH, et al. Insulin stimulation of gene expression mediated by p21 ras activation. EMBO J 1991; 10: 1103–9.
Bos JL. The ras gene family and human carcinogenesis. Mut Res 1988; 195: 255–71.
Bjorntorp P. Metabolic implications of body fat distribution. Diabetes Care 1991; 14: 1132–43.
Kissebah AH, Vydelingum N, Murray R, et al. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab 1982; 54: 254–60.
Krotkiewski M, Bjorntorp P, Sjostrom L, Smith U. Impact of obesity on metabolism in men and women: importance of regional adipose distribution. J Clin Invest 1983; 72: 1150–62.
Donahue RP, Abbott RD, Bloom E, Reed DM, Yano K. Central obesity and coronary heart disease in men. Lancet 1987; 1: 882–4.
Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity and risk for colon cancer and adenoma in men. Ann Intern Med, in press..
Krotkiewski M, Bjorntorp P, Sjostrom L, Smith U. Impact of obesity on metabolism in men and women. J Clin Invest 1983; 72: 1150–62.
Koivisto VA, Yki-Yarvinen H, DeFronzo RA. Physical training and insulin sensitivity. Diabetes Metab Rev 1988; 1: 445–81.
Regensteiner JG, Mayer EJ, Shetterly SM, et al. Relationship between habitual physical activity and insulin levels among nondiabetic men and women. Diabetes Care 1991; 14: 1066–74.
Dowse GK, Zimmet PZ, Gareeboo H, et al. Abdominal obesity and physical inactivity are risk factors for NIDDM and impaired glucose tolerance in Indian, Creole and Chinese Mauritians. Diabetes Care 1991; 14: 271–82.
Lindgarde F, Saltin B. Daily physical activity, work capacity and glucose tolerance in lean and obses normoglycaemic middle-aged men. Diabetologia 1981; 20: 134–8.
Wang JT, Ho LT, Tang KT, Wang LM, Chen YDI, Reaven GM. Effect of habitual physical activity on agerelated related glucose intolerance. J Am Geriatr Soc 1989; 37: 203–9.
DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose: results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 1981; 30: 1000–7.
Lilloja S, Mott DM, Zawadzki JK, Young AA, Abbott WG, Bogardus C. Glucose storage is a major determinant of in vivo “insulin resistance” in subjects with normal glucose tolerance. J Clin Endocrinol Metab 1986; 62: 922–7.
Shulman GI, Rothman DL, Jue T, Stein P, DeFronzo RA, Shulman RG. Quantitation of muscle glycogen synthesis in normal subjects and subjects with noninsulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med 1990; 322: 223–8.
Hamman RF. Genetic and environmental determinants of non-insulin-depeendent diabetes mellitus (NIDDM). Diabetes/Metabol Res 1992; 8: 287–338.
Helmrich SP, Ragland DR, Leung RW, Paffenbarger RS. Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. N Engl J Med 1991; 325: 147–52.
Manson JE, Rimm EB, Stampfer MJ, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 1991; 238: 774–8.
Manson JE, Nathan DM, Krolewski AS, Stampfer MJ, Willett WC, Hennekens CH. A prospective study of exercise and incidence of diabetes among US male physicians. JAMA 1992; 268: 63–7.
DeFronzo RA, Bonadonna RC, Ferranini E. Pathogenesis of NIDDM. Diabetes Care 1992; 15: 318–68.
Schneider SH, Amorosa LF, Khachadurian AK, Ruderman NB. Studies on the mechanism of improved glycose control during regular exercise in type 2 (non-insulin dependent) diabetes. Diabetologia 1984; 26: 355–60.
Burstein R, Polychronakos C, Toews CJ, MacDougall JD, Guyda HJ, Posner BI. Acute reversal of the enhanced insulin action in trained athletes: associations with insulin receptor changes. Diabetes 1985; 34: 756–60.
Blair SN, Kohl HW, Gorden NF, Paffenbarger RS. How much physical activity is good for health? Annu Rev Public Health 1992; 13: 99–126.
Kriska AM, Bennett PH. An epidemiologic perspective of the relationship between physical activity and NIDDM: from activity assessment to intervention. Diabetes/Metab Rev 1992; 8: 355–72.
Hagve TA. Effects of unsaturated fatty acids on cell membrane functions. Scand J Clin Lab Invest 1988; 48: 381–8.
Yorek M, Leeney E, Dunalp J, Ginsberg B. Effect of fatty acid composition on insulin and IGF-I binding in retinoblastoma cells. Invest Ophthalmol Vis Sci 1989; 30: 2087–92.
Ginsberg BH, Chaterjee P, Yorek M. Increased membrane fluidity is associated with greater sensitivity to insulin (Abstract). Diabetes 1987; 36: Suppl 1: 51A..
Ginsberg BH, Jabour J, Spector AA. Effect of alterations in membrane lipid unsaturation on the properties of the insulin receptor of Ehrlich ascites cells. Biochem Biophys Res Commun 1981; 103: 219–26.
Field CJ, Ryan EA, Thomson ABR, Clandinin MT. Dietary fat and the diabetic state alter insulin binding and the fatty acyl composition of the adipocyte plasma membrane. Biochem J 1988; 253: 417–24.
Field CJ, Ryan EA, Thomson ABR, Clandinin MT. Dietary fat composition alters membrane phospholipid composition, insulin binding, and glucose metabolism in adipocytes from control and diabetic animals. J Biol Chem 1990; 265: 11143–50.
Storlien LH, Kenkins AB, Chislom DJ, Pascoe WS, Khouri S, Kraegen EW. Influence of dietary fat composition on development of insulin resistance in rats: relationship to muscle triglyceride and w-3 fatty acids in muscle phospholipid. Diabetes 1991; 40: 280–9.
Pelikanova T, Kohout M, Valek J, Base J, Kazdova L. Insulin secretion and insulin action related to the serum phospholipid fatty acid patterrn in health men. Metabolism 1989; 38: 188–92.
Ruiz-Gutierrez V, Stifel P, Villar J, Garcia-Donas MA, Acosta D, Carneado J. Cell membrane fatty acid composition type 1 (insulin-dependent) diabetic patients: relationship with sodium transport abnormalities and metabolic control. Diabetologia 1993; 36: 850–6.
Borkman M, Storlien LH, Pan DA, et al. The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids. N Engl J Med 1993; 328: 238–44.
Colditz GA, Manson JE, Stampfer MJ, Rosner B, Willett WC, Speizer FE. Diet and risk of clinical diabetes in women. Am J Clin Nutr 1992; 55: 1018–23.
Marshall JA, Hamman RF, Baxter J. High fat, low carbohydrate diet and the etiology on non-insulin-dependent diabetes mellitus: the San Luis Valley Diabetes Study. Am J Epidemiol 1991; 134: 590–603.
Marshall JA, Hoag S, Jones RH, Hamman RF. Relationships between dietary long chain omega-3 fatty acids, physical activity and fasting insulin levels among persons without diabetes: the San Luis Valley Diabetes Study. 14th IDF Congress, Nutr Satel, June 1991..
Hunter DJ, Rimm EB, Sacks FM, et al. Comparison of measures of fatty acid intake by subcutaneous fat aspirate, food frequency questionnaire, and diet records in a free-living population of US men. Am J Epidemiol 1992; 135: 418–27.
Linscheer WG, Vergroesen AJ. Lipids. In. Shils M, Young VR, eds. Modern Nutrition in Health and Disease, 7th7th Ed., Philadelphia, PA (USA): Lea & Febiger, 1988: 72–107.
Deibert DC, DeFronzo RA. Epinephrine-induced insulin resistance in man. J Clin Invest 1980; 65: 717–21.
Lager I, Atvall S, Eriksson BM, von Schenk H, Smith U. Studies on the insulin-anatagonistic effect of catecholamines in normal man. Diabetologia 1986; 29: 409–16.
Surwit RS, Feinglos MN. Stress and autonomic nervous system in type II diabetes. A hypothesis. Diabetes Care 1988; 11: 83–5.
Surwit RS, Schneider MS, Feinglos MN. Stress and diabetes mellitus. Diabetes Care 1992; 15: 1413–22.
Kune S, Kune GA, Watson LF, Rahe RH. Recent life change and large bowel cancer. data from the Melbourne colorectal cancer study. J Clin Epidemiol 1991; 44: 57–68.
Courtney JG, Longnecker MP, Theorell T, Gerhardssonde Verdier M. Stressful life events and the risk of colorectal cancer. Epidemiology 1993; 4: 407–14.
Wolever TMS, Jenkins DJA, Jenkins AL, Josse RG. The glycemic index: methodology and clinical implications. Am J Clin Nutr 1991; 54: 846–54.
Trout DL, Behall KM, Osilesi O. Prediction of glycemic index for starchy foods. Am J Clin Nutr 1993; 58: 873–8.
Schoch TJ, Maywald EC. Preparation and properties of various legume starches. Cereal Chem 1976; 45: 564–73.
Wolever TMS. The glycemic index. In: Bourne GH, Ed. Aspects of Some Vitamins, Minerals and Enzymes in Health and Disease. Basel, Switzerland: Karger, 1990: World Rev Nutr Diet Vol 62: 120–85.
Goddard MS, Young G, Marcus R. The effect of amylose content on insulin and glucose responses to ingested rice. Am J Clin Nutr 1984; 39: 388–92.
Jenkins DJA, Jenkins AL, Rao AV, Thompson LU. Starchy foods, type of fiber, and cancer risk. Prev Med 1987; 16: 545–53.
Riccardi G, Rivellese AA. Effects of dietary fiber and carbohydrate on glucose and lipoprotein metabolism in diabetic patients. Diabetes Care 1991; 14: 1115–25.
Gerhardsson de Verdier M, Longncker MP. Eating frequency-a neglected risk factor for colon cancer? Cancer Causes Control 1991; 3: 77–81.
Manolio TA, Savage PJ, Burke GL, et al. Correlates of fasting insulin levels in young adults: the CARDIA study. J Clin Epidemiol 1991; 44: 571–8.
Wynder EL, Kajitani T, Ishikawa S, et al. Environmental factors of cancer of the colon and rectum. II. Japanese epidemiological data. Cancer 1969; 23: 1210–20.
Steinmetz KA, Potter JD. Food-group consumption and colon cancer in the Adelaide Case-Control Study. I. Vegetables and fruit. Int J Cancer 1993; 53: 711–19.
Zaridze D, Filipchenko V, Kustov V, Serdyuk V, Duffy S. Diet and colorectal cancer. results of two case-control studies in Russia. Eur J Cancer 1992; 29A: 112–5.
Garg A, Bantle JP, Henry RR, et al. Effects of varying carbohydrate content of diet in patients with non-insulin-dependent diabetes mellitus. JAMA 1994; 271: 1421–8.
Thornton JR, Dryden A, Kelleher J, Losowsky MS. Super-efficient starch absorption. A risk factor for colonic neoplasia? Dig Dis Sci 1987; 32: 1088–91.
Dyer RG. The changing shape of obesity, Japan 1990. Diabetic Med 1991; 8 492–4.
Sasaki A, Kamado K, Uehara M. A chnging pattern of causes of death among diabetics during a 25-year period in the Osaka District, Japan. Diabetes Res Clin Practice 1991; 13: 213–20.
Tuomilehto J, Knowler WC, Zimmet P. Primary prevention of non-insulin-dependent diabetes mellitus. Diabetes Metab Rev 1992; 8: 339–53.
Potter JD, Slattery ML, Bostick RM, Gapstur SM. Colon cancer. A review of the epidemiology. Epidemiol Rev 1993; 15: 499–545.
Sutton TD, Eide TJ, Jass JR. Trends in colorectal cancer incidence and histologic findings in Maori and Polynesian residents of New Zealand. Cancer 1993; 71: 3839–45.
Smith AH, Pearce NE, Joseph JG. Major colorectal cancer aetiological hypotheses do not explain mortality trends among Maori and non-Maori New Zealanders. Int J Epidemiol 1985; 14: 79–85.
Simmons D, Baker J, James A, Roberts A. Has the process causing noninsulin dependent diabetes started at birth? Evidence in neonates from a population with a high prevalence of diabetes. NZ Med J 1992; 105: 326–8.
McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet 1991; 337: 382–6.
Aronoff SL, Bennett PH, Gorden P, Rushforth N, Miller M. Unexplained hyperinsulinemia in normal; and “prediabetic” Pima Indians compared with normal caucasians. Diabetes 1977; 26: 827–40.
King H, Finch C, Zimmet P, Almers M. Plasma glucose and insulin responses in young Papua New Guineans (aged 10–19 years). Diab Res Clin Prac 1990; 10: 153–9.
Joffe BI, Panz VR, Wing JR, Raal FJ, Seftgel HC. Pathogenesis of non-insulin-dependent diabetes mellitus in the black population of southern Africa, Lancet 1992; 340: 460–2.
Zamboni M, Armellini F, Cominacini L. et al. Obesity and regional body-fat distribution in men: separate and joint relationships to glucose tolerance and plasma lipoproteins. Am J Clin Nutr 1994; 60: 682–7.
Evans DJ, Hoffman RG, Kalkhaff RK, Kissebah AH. Relationship of body fat topography to insulin sensitivity and metabolic profiles in premenopausal women. Metabolism 1984; 33: 68–75.
Peiris AN, Mueller RA, Struve MF, Smith GA, Kissebah AH. Relationship of androgenic activity to splanchnic insulin metabolism and peripheral glucose utilization in premenopausal women. J Clin Endocrinol Metab 1987; 64: 162–9.
Bjorntorp P. Abdominal obesity and the development of non-insulin-dependent diabetes. Diabetes/Metab Rev 1988; 4: 615–22.
Williams JC, Walsh DA, Jackson JF. Colon carcinoma and diabetes mellitus. Cancer 1984; 54: 3070–1.
La Vecchia C, D'Avanzo B, Negri E, Franceschi S. History of selected diseases and the risk of colorectal cancer. European J Cancer 1991; 27: 582–6.
Adami HO, McLaughlin J, Ekbom A, et al. Cancer risk in patients with diabetes mellitus. Cancer Causes Control 1991; 2: 307–14.
McKeown-Eyssen G. The epidemiology of colorectal cancer revisited: are serum trglycerides and/or plasma glucose associated with risk. Cancer Epi Biomakkers (in press)..
Hymans DE. Gastrointestinal problems in the old. Br Med J 1974; 1: 107–110.
Milne JS, Williamson J. Bowel habits in older people. Gerontol Clin 1972; 14: 56–60.
Bingham SA, Cummings JH. Effect of exercise and physical fitness on large bowel intestinal function. Gastroenterol 1989; 97: 1389–99.
Oettle GJ. Effect of mpderate exercise on bowel habit. Gut 1991; 32: 941–3.
Coene C, Wegener M, Wedmann B, Schmidt G, Hoffmann S. Does physical exercise influence bowel transit time in healthy young men? Am J Gastroenterol 1992; 87: 292–5.
Koffler KH, Menkes A, Redmond RA, Whitehead WE, Pratley RE, Hurley BF. Strength training accelerates gastrointestinal transit in middle-aged and older men. Med Sci Sports Exercise 1991; 24: 415–9.
Sugimura T, Sato S. Mutagens-carcinogens in foods. Cancer Res 1983; 43: 2415s-21s.
Freudenheim JL, Graham S, Horvath PJ, Marshall JR, Haughey BP, Wilkinson G. Risks associated with source of fiber and fiber components in cancer of the colon and rectum. Cancer Res 1990; 50: 3295–300.
Hu K, Liu Y, Yu Y,, et al. Diet and cancer of the colon and rectum: a case-control study in China. Int J Epidemiol 1991; 20: 362–7.
Author information
Authors and Affiliations
Additional information
This research was supported by research grant number CA 55075 from the US National Institutes of Health, and Special Institution Grant No. 18 from the American Cancer Society.
Rights and permissions
About this article
Cite this article
Giovannucci, E. Insulin and colon cancer. Cancer Causes Control 6, 164–179 (1995). https://doi.org/10.1007/BF00052777
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00052777