Article Text
Abstract
Background Socioeconomic status (SES) has long been conjectured to be associated with the incidence and progression of chronic kidney disease (CKD), but few studies have examined this quantitatively. This meta-analysis aims to fill this gap.
Methods A systematic literature review was performed using Medline and EMBASE to identify observational studies on associations between SES and incidence and progression of CKD, published between 1974 and March 2017. Individual results were meta-analysed using a random effects model, in line with Meta-analysis of Observational Studies in Epidemiology guidelines.
Results In total, 43 articles met our inclusion criteria. CKD prevalence was associated with several indicators of SES, particularly lower income (OR 1.34, 95% CI (1.18 to 1.53), P<0.001; I2=73.0%, P=0.05); lower education (OR 1.21, 95% CI (1.11 to 1.32), P<0.001; I2=45.20%, P=0.034); and lower combined SES (OR 2.18, 95% CI (1.64 to 2.89), P<0.001; I2=0.0%, P=0.326). Lower levels of income, occupation and combined SES were also significantly associated with progression to end-stage renal disease (risk ratio (RR) 1.24, 95% CI (1.12 to 1.37), P<0.001; I2=66.6%, P=0.006; RR 1.05, 95% CI (1.01 to 1.09), P=0.012; I2=0.0%, P=0.796; and RR 1.39, 95% CI (1.09 to 1.79), P=0.009; I2=74.2%, P=0.009). Subgroup analyses generally confirmed these results, except in a few cases, such as an inverse association related to particular socioeconomic backgrounds and where results were adjusted by more disease-related risk factors.
Conclusion Lower income was most closely associated with prevalence and progression of CKD, and lower education was significantly associated with its prevalence. Evidence for other indicators was inconclusive.
- socioeconomic status
- epidemiology of chronic non-communicable diseases
- health inequalities
Statistics from Altmetric.com
Introduction
Chronic kidney disease (CKD) has become a global issue because of its rapidly increasing prevalence and cost. Its worldwide prevalence ranges from 10.2% to 13%,1–3 and middle-aged and older people with a history of hypertension or diabetes are more susceptible.4 Individuals with lower socioeconomic status (SES) may suffer from unrecognised and untreated CKD as well as end-stage renal disease in both low-income and middle-income countries and developed countries.5–7 This may be because of poor access to healthy diets, physical activity, health information and quality healthcare.8 9
Studies on the overall impact of SES on CKD have obvious limitations. SES is a multidimensional concept incorporating material and social factors. These can differ substantially in their associations and effect size.10 In the absence of a uniform definition of SES, various substitutes have been used, including income, education level, occupation, wealth or geographic location. Second, many studies have been confined to particular regions, so the results may not be generalisable. Studies have therefore provided inconsistent results about the magnitude of associations, making it hard to understand the true association between SES and CKD in the general population. Vart et al 5 performed a meta-analysis to explore the association between the two, combining estimates from different socioeconomic indicators. However, the mechanisms underlying the association between individual indicators and the onset and progression of CKD need further investigation. We therefore carried out a meta-analysis to examine the association between CKD and several individual indicators of SES.
Materials and methods
Study identification
Information source and search strategy
Eligible studies on associations between SES and CKD were found by searching four electronic databases: PubMed/Medline, OV/EMBASE, Cochrane Library and Chinese Biomedicine Database from 1974 to March 2017.
Suitable studies involved associations between individual indicators (income, educational attainment or occupation) or a combined index of SES and CKD.11 12 Keywords included ‘social class’, ‘socioeconomic status, position, factors’, ‘income’, ‘education level’, ‘occupations’, ‘chronic kidney disease’, ‘chronic renal insufficiency’, ‘chronic kidney failure’ and ‘chronic renal dysfunction’ (see online supplementary material/search strategy). There were no restrictions on languages or countries of publication. Unpublished or non-peer-reviewed articles were excluded. The review complied strictly with Meta-analysis of Observational Studies in Epidemiology guidelines for meta-analyses13 (online supplementary table 1).
Supplementary file 1
Selection criteria
Studies were independently screened by two reviewers (JL and ST) using the following criteria:
Inclusion criteria: (1) prospective, retrospective and cross-sectional observational studies; (2) adult population or adult patients diagnosed with CKD; (3) reported associations between at least one determinant of SES and CKD, using adjusted HR, risk ratio (RR) or OR with 95% CIs or sufficient information to calculate these statistics.
Exclusion criteria: (1) abstracts, protocols, letters, expert opinions, case reports and reviews; (2) studies on acute renal failure or unrepresented CKD.
Any disagreements were resolved through discussion with another reviewer (XZ).
Data abstraction
Two independent reviewers (XZ and JL) extracted information about each article including the first author’s name, year of publication, country where the study was conducted, type of study design, covariate adjustment degree, sample size, duration of study, indicators of SES (income, education, occupation, combined SES), development and progression of CKD, mean age, sex and risk estimates (OR or RR) with corresponding 95% CIs.
Measurements of the indicators of SES were all categorised (dichotomised or multicategorised). Combined SES was an indicator which incorporated more than one individual SES indicator. It could be a comprehensive indicator determined by income, education and occupation,14 by Index of Multiple Deprivation (IMD) at practice level,15–17 or by summary score of area-level SES constructed summing z scores 6–7 census-derived SES indicators.18–20 Outcomes were not restricted, but included prevalence, incidence and progression of CKD. To augment between-study comparability using different indicator categories, we also compared the lowest and highest SES categories. The national income level was classified into high, middle or low using the World Bank’s 2003 World Development Indicator.21 The degree of adjustment was categorised as ‘minimal’ or ‘maximal’ depending on whether a model used three or fewer (age, gender or ethnicity), or more than three control covariates.11 22
Quality assessment
The quality of studies was assessed using the Newcastle-Ottawa Quality Assessment Scale for cohort or case–control studies, and the Cross-Sectional/Prevalence Study Quality Assessment recommended by the Agency for Healthcare Research and Quality for cross-sectional studies. For Newcastle-Ottawa, the maximum numbers of points awarded in the selection, comparability and exposure (for cohort studies) or outcome (for case–control studies) categories were 4, 2 and 3. The Cross-Sectional/Prevalence Study Quality Assessment contains 11 items covering information source, subject quality, study design and outcome completeness. Each item has ‘Yes/No/Unclear’ response options: ‘Yes’ scored one point and ‘No’ or ‘Unclear’ zero, and the scores were summed (online supplementary tables 2–4). There is no agreed level of study quality, so we rated it as ‘High’, ‘Moderate’ or ‘Low’, if it had values of 7–9, 4–6 and 0–3 for cohort or case–control studies, and 8–11, 5–7 and 0–4 for cross-sectional studies.
Statistical analysis
The estimated associations were obtained using either logistic regressions or Cox proportional hazards models with reported adjusted ORs, HRs or RRs. For studies8 18 20 23–26 reporting separate estimates by gender, the risk estimates were pooled (weighted by the inverse of the variance) to obtain summarised estimates.
The meta-analyses used the DerSimonian and Laird27 random effects model, which takes into account within-study and between-study variations, stratified by study design28 (cohort, case–control or cross-sectional studies). We used adjusted OR and 95% CIs as metrics for pooled estimates in case–control or cross-sectional studies, and RR and 95% CIs in cohort studies. To evaluate the heterogeneity, we used Cochrane’s Q test. This is statistically significant if P<0.1; I2 below 30% is defined as unimportant, 30%–50% as moderate, 50%–75% as substantial and >75% as considerable heterogeneity.29 30
We also used subgroup analyses by geographic area, national income level, different degrees of adjustment for important disease-related risk factors (eg, comorbid conditions, access to healthcare and health behaviours) based on studies that had maximum adjustment, study design, study quality and estimated glomerular filtration rate (eGFR) calculation equation (only for incidence). ORs or RRs were compared using the Q test to assess the difference.
To evaluate the stability of the results and to test whether a study had excessive influence on the final result, we used a leave-one-study-out sensitivity analysis,31 especially for pooled studies with considerable heterogeneity. The presence of publication bias for the hypothesis of an association between low SES and CKD was assessed by funnel plots, coupled by Egger’s regression asymmetry test32 and Begg’s adjusted rank correlation test.33 The statistical software was Stata V.13 (Stata, College Station, Texas, USA), and a two-sided P<0.05 was considered statistically significant in all tests.
Results
Search results
In total, 3140 articles were identified from electronic databases. After removing duplicates, 2142 unique articles remained, of which 989 did not address the issue of interest, and 898 were not related to the incidence and progression of CKD, leaving 43 articles that met our selection criteria and were therefore included in our meta-analysis (online supplementary figure 1).
The mean age of participants in the studies ranged from 39.7 to 72.7 years. The studies took place in America, Europe, Asia and Africa. Seventeen articles defined CKD as eGFR<60 mL/min/1.73 m2, as in the CKD-Modification of Diet in Renal Disease Study.8 14 17 26 34–46 Eight articles used Epidemiology Collaboration (EPI),15 24 47–52 one used Cockcroft-Gault normalised to body surface area equation,53 two used creatinine level25 54 and the rest eGFR<45 mL/min/1.73 m2.55
A total of 29 articles8 14 15 17 24–26 35–38 40–57 focused on associations between SES and prevalence and incidence of CKD, with a total of 584 805 participants. The majority were cross-sectional studies (n=21) on the association between SES and CKD prevalence. Nineteen studies8 15 17 24 35–38 40–42 44 45 47 49–51 54 57 were of moderate quality, nine14 25 26 43 46 52 53 55 56 high and only one48 low (online supplementary tables 2–4). Fourteen articles16 58–66 examined the relationship between SES and CKD progression, across more than 6 978 082 participants (two articles60 65 did not provide the number of participants). Of these, six studies7 16 60 63–65 were of moderate quality, and eight18–20 23 58 60–62 high. Table 1 shows the characteristics of the studies on prevalence, incidence and progression of CKD. The between-researcher agreement levels on the quality of cross-sectional, case–control and cohort studies were 19/21, 4/5 and 15/17, respectively. The final quality assessments are shown in online supplementary tables 2–4.
Characteristics and results of included studies on incidence and progression of CKD
Overall results
Associations of SES with CKD prevalence and incidence
A total of 21 articles14 15 17 24 35–38 40–44 47–49 51–53 56 57 reporting 24 cross-sectional studies (two articles43 52 reported five of these), and conducted in the USA,15 35–38 43 51 52 56 57 Europe,14 17 24 48 49 52 Asia,40 41 43 44 Africa,42 Mexico,53 Brazil47 and Australia43 were published between 2003 and 2016. Most studies focused on associations between CKD prevalence and income (n=17) or education (n=14). Significant associations were found between prevalence and most indicators of SES: lower income (OR 1.34, 95% CI (1.18 to 1.53), P<0.001; I2=73.0%, P=0.05); lower education (OR 1.21, 95% CI (1.11 to 1.32), P<0.001; I2=45.20%, P=0.034); and lower combined index (OR 2.18, 95% CI (1.64 to 2.89), P<0.001; I2=0.0%, P=0.326) (figure 1A–D). Lower level occupations were not associated with prevalence (OR 1.09, 95% CI (0.96 to 1.23), P=0.168; I2=26.4%, P=0.227).
Associations between socioeconomic status (SES) and chronic kidney disease (CKD) prevalence. (Parts A–D demonstrate different associations between SES indicators and CKD incidence in the form of lower income, education level, occupation status and combined SES, respectively.)
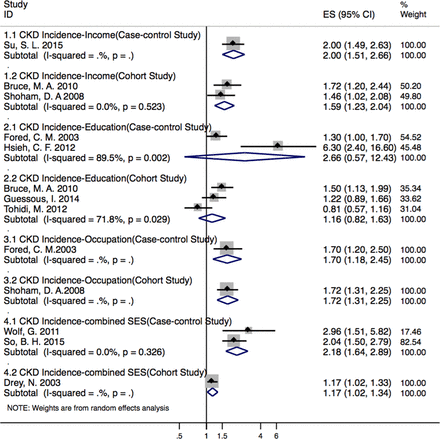
Five cohort studies8 26 50 54 55 and three case–control studies25 45 46 explored the relationship between SES and CKD incidence. Incidence was significantly associated with lower income (RR 1.59, 95% CI (1.23 to 2.04), P<0.01; I2=0.0%, P=0.5/OR 2.00, 95% CI (1.49 to 2.60), P<0.001; n=1), occupation level (RR 1.72, 95% CI (1.31 to 2.25), P<0.01; n=1/OR 1.70, 95% CI (1.18 to 2.45), P=0.005; n=1) and combined index (RR 1.17, 95% CI (1.12 to 1.23), P<0.01; n=1/OR 2.18, 95% CI (1.64 to 2.89), P=0.003), but had no association with lower educational level (RR 1.16, 95% CI (0.82 to 1.63), P=0.4; I2=71.8%, P=0.03/OR 2.66, 95% CI (0.57 to 12.43), P=0.212; I2=89.5%, P=0.002) (figure 2).
Associations between socioeconomic status (SES) and chronic kidney disease (CKD) incidence (by different study designs with each SES indicator). ES, effect size.
The association between SES and CKD progression
Twelve cohort studies7 16 18–20 23 58 60–62 65 66 provided RRs for the association between CKD progression and indicators of SES, mostly income (n=7) or education (n=7). Progression was significantly associated with lower income (RR 1.24, 95% CI (1.12 to 1.37), P<0.001; I2=66.6%, P=0.006), lower level occupation (RR 1.05, 95% CI (1.01 to 1.09), P=0.012; I2=0.0%, P=0.796) and lower combined SES (RR 1.39, 95% CI (1.09 to 1.79), P=0.009; I2=74.2%, P=0.009). There was no significant association with education (RR 1.11, 95% CI (0.94 to 1.30), P=0.218; I2=71.3%, P=0.002) (figure 3A–D). Two case–control studies63 64 showed significant associations between lower income and CKD progression (OR 3.83, 95% CI (2.28 to 6.42), P<0.001; I2=30.0%, P=0.232). No single studies exerted an obviously excessive influence on the associations.
Associations between socioeconomic status (SES) and chronic kidney disease (CKD) progression to end-stage renal disease (ESRD). (Parts A–D demonstrate different associations between SES indicators and CKD progression in the form of lower income, education level, occupation status and combined SES, respectively.) RR, risk ratio.
Subgroup analyses
The associations between CKD prevalence and progression and the indicators of SES varied across several factors (see table 2). We also planned to use gender but there were insufficient data. When relative estimations were fully adjusted for comorbid conditions, health access and health-related behaviours, the associations between CKD prevalence and lower income and education level were still significant, with lower heterogeneity (income: OR 1.46, 95% CI (1.23 to 1.74), P<0.001; I2=49.4%, P=0.139; education: OR 1.11, 95% CI (1.03 to 1.20), P=0.008; I2=0.0%, P=0.398). All the significant associations between lower income, education and occupation and SES prevalence were observed in high-income (income: OR 1.49, 95% CI (1.32 to 1.67), P<0.001; I2=50.1%, P=0.024; education: OR 1.19, 95% CI (1.06 to 1.34), P=0.003; I2=40.7%, P=0.120; occupation: OR 1.21, 95% CI (1.06 to 1.38), P=0.004; I2=0.0%, P=0.849), but not upper middle-income countries (income: OR 1.20, P=0.340; education: OR 1.28, P=0.163; occupation: OR 0.91, P=0.293). The association between prevalence and education was similar in the USA, Europe and Asia-Pacific Region (OR=1.17, 1.18, 1.21; P=0.783), but the association between prevalence and lower income was more marked in the USA than Europe (OR=1.55 vs 1.14; P=0.013). The results of studies from the 2000s and 2010s were similar (comparison of ORs from subgroups of 2000s vs 2010s in income (P=0.809), in education (P=0.974) and occupation (P=0.353)).
Pooled OR (from cross-sectional) of CKD prevalence in the lower SES indicators compared with the higher in series of subgroup analyses
All the cohort studies on the association between SES and disease progression were conducted in high-income countries, and there was a significant association between lower income and progression in several geographic areas (USA: RR 1.27, 95% CI (1.08 to 1.50), P=0.004; European countries: RR 1.19, 95% CI (1.13 to 1.26), P<0.001). The association between lower educational attainment and disease progression in Europe (RR 1.23, 95% CI (1.17 to 1.30), P<0.001; I2=0.0%, P=0.861) was statistically significant but inconsistent with the overall trend. If the analysis was limited to studies that fully adjusted for disease-related risk factors, the associations between progression and lower income and education level were insignificant (OR 1.29 vs 1.06, P=0.276 vs 0.742). More studies were published after 2010, accounting for more than half of the eligible studies on both income and education, with similar results (RR 1.39, 95% CI (1.11 to 1.74), P=0.004; RR 1.07, P=0.454) with substantial heterogeneity (I2=75.0%, P=0.007; I2=68.1%, P=0.014). (See table 3.)
Pooled RR (from cohort studies) of CKD progression in the lower SES compared with the higher in series of subgroup analyses
The publication bias funnel plots and results of Begg’s test and Egger’s test (online supplementary figures 2.1–2.3, 3.1, 3.2) showed no publication bias except for studies on the association between income and disease progression (Egger’s test P=0.05). Publication bias analysis was not possible on other indicators of SES because of the limited number of studies.67
Discussion
This meta-analysis has shown several associations between individual indicators of SES and CKD prevalence and progression. The effect sizes of these associations varied by national income, geographic location and level of adjustment. Lower income and education level were strongly associated with CKD prevalence in high-income countries, except Europe. Disease progression was associated with lower income in the USA and Europe, but the association with lower educational attainment was only significant in Europe.
Interactions between indicators of SES may bring statistical artefacts, especially for parameters with significant associations such as income and education level. A previous study clarified that indicators of SES are only modestly correlated with each other, and we found that income was still associated with CKD prevalence even after full adjustment for other indicators. Indicators of SES are therefore not directly comparable and may be independently associated with health outcomes to some degree.
The association between lower income and CKD prevalence could be attributed to food insufficiency, inadequate nutritional intake, exposure to environmental toxins, infection and/or inflammation, distress or anxiety over income disadvantage, inadequate health insurance and poorer access to quality healthcare services.15 43 53 56 Inadequate diet and unhealthy lifestyles were likely to be associated with obesity, diabetes mellitus and hypertension, which may be causally linked to kidney disease.35 68 There was a significant association in high-income but not upper middle-income countries. This might be partly explained by differences in healthcare and insurance systems.25 Socialised medicine systems in some upper middle-income countries might attenuate the association between income and CKD. Income-related and education-related inequalities might also be smaller in countries providing relatively generous universal welfare, such as Scandinavian countries.69 The effect size was larger in the USA than in Europe, which might be partly because of stricter guidelines on comorbidity management in Europe,70 and a publicly financed healthcare system in most European Union member states.71
The association between lower educational attainment and CKD was complex, as it may be mediated by behavioural risk factors. For example, several studies72–74 have found that lower education is linked to various CKD-related behavioural risk factors (smoking and alcohol, poor diet planning ability and lack of physical activity), and chronic conditions leading to secondary CKD, such as diabetes and hypertension. Better education enables individuals to make better healthcare decisions and obtain better access to healthcare interventions and plans,75 so helps to improve general health in individuals and their children.37 Interestingly, awareness of CKD is not linked to education level. For example, one study35 found that the majority of subjects with more than high school education were unaware of their CKD status
Only a few studies have examined the association between occupation and CKD, and occupation categories were not standard, but each OR or RR maximised the comparability. Individuals with lower level occupations were more likely to be exposed to hazardous working conditions,25 and blue collar workers were more likely to be obese than white collar workers.68 76 77 Obesity is a significant risk factor for diabetes and hypertension,78 and in turn to CKD. The potential mechanisms linking lower level occupations to CKD onset included fewer nephrons, nephrotoxins (analgesics), and poor diet and health behaviours.55 Finally, social status itself might confer health benefits, possibly via psychosocial mechanisms, regardless of economic elements.79
Our review was rigorous in maximising its completeness and quality of evidence. To explore potential confounders, we conducted subgroup and sensitivity analyses to distinguish and diminish heterogeneity. Substantial heterogeneities were detected across the studies analysed and could not be effectively eliminated even in subgroups. The heterogeneities across countries may have been because of differences in economic or healthcare systems, and income distribution. This paper is the first attempt, to our knowledge, to include all the specific determinants of SES and elements of CKD when studying the associations between these two issues. It is in line with the view that association studies should not rely on just one indicator of SES, as each one represents a different causal process or pathway and they should not be used interchangeably.80 The population in our meta-analysis covered more geographic areas, national income levels and CKD definitions than the previous meta-analysis.5 We also adjusted the results for CKD-related healthcare access and health-related behaviours to explore clearer associations and possible mechanisms than socioeconomic indicators alone could provide. Our study reflects the global population (North America, Europe, Asia-Pacific Region, Latin America and Africa), including regions with different economic and social development levels (developed countries and low-income and middle-income countries).
This paper has several limitations. First, income, education level, occupation and the combined index were defined and classified differently in the studies analysed. Second, the definition of CKD also varied, which may lead to overdispersion of the estimated effects. Third, there might have been some selection bias in the study samples. For example, in some studies, subjects were recruited from enterprises or factories that offered physical examinations for employees. These subjects might therefore have better overall health than the general population. Finally, few studies explored the association between occupation and CKD, or with CKD incidence as an outcome.
Most studies on the association between SES and CKD prevalence were cross-sectional and not fully adjusted for disease-related risk factors including access to healthcare (insurance or routine healthcare visits), and health-related behaviours other than smoking and alcohol consumption (such as diet, physical activity or sedentary time). The case–control or cohort studies often assessed exposure and covariates just once during follow-up, and did not fully capture the biological mechanism governing disease progression. This warrants more exploration of the changes in comorbid conditions and figures set as outcomes, and the association between continuous variables.
Conclusion
Several individual indicators of SES were associated with the prevalence and progression of CKD. Lower income was associated with prevalence and progression, but the effects of education, occupation and overall status were inconsistent. Risk estimates differed by national income levels, geographic locations and adjustment level. Our findings may be useful in developing more effective CKD prevention programme among socioeconomically disadvantaged populations.
What is already known on this subject
Individuals with lower socioeconomic status may be more likely to suffer from chronic kidney disease (CKD). This disease is one of the major public health concerns of the 21st century because of its high prevalence, mortality and social cost. Previous studies have obvious limitations including vague and variable definitions of socioeconomic status, because of the multidimensional nature of the concept, and biased results that cannot be generalised more widely because of country-specific and region-specific socioeconomic background.
What this study adds
This study is a first effort to quantitatively evaluate associations between CKD and key indicators of socioeconomic status, including income, educational attainment, occupation and a comprehensive index. Subgroup and sensitivity analyses were used to explore how associations were affected by other factors, including study locations and times, adjustment for other factors and national economic background. These may help in developing more effective kidney disease prevention programme for disadvantaged populations.
References
Footnotes
XZ and JL contributed equally.
Contributors XZ and JL conceived the study. JL and ST extracted the data. XZ, JL and HGH analysed the results and drafted the manuscript. HGH and YL assisted with the statistical analyses and edited the manuscript. YL and PF refined the study design and contributed to supervision. Each author contributed important intellectual content during the manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Funding This work was partially supported by the international cooperation project (2016HH0069) funded by the Science and Technology Department of Sichuan Province, China.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.